Research on propene oligomerization reaction over the Fenton's reagent modified ZSM-5
-
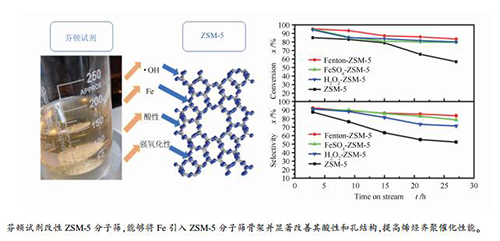
摘要: 采用浸渍法分别制备了芬顿试剂及其原料FeSO4和H2O2改性的ZSM-5分子筛,并与未改性的母体ZSM-5分子筛进行了对比实验。通过一系列表征手段及丙烯齐聚反应考察了各催化剂的理化性质和催化性能。结果表明,芬顿试剂、FeSO4、H2O2的改性处理均会引起ZSM-5分子筛脱铝,从而导致其硅铝比升高。但与FeSO4改性相比,芬顿试剂由于其产生的大量羟基自由基更容易将Fe引入分子筛,形成新的活性中心,同时芬顿试剂改性还能使分子筛的比表面积和介孔的体积增大,调节催化剂的酸性。与母体ZSM-5相比,Fenton-ZSM-5催化剂具有优异的催化活性和稳定性。初始丙烯转化率和柴油选择性分别高达98.3%和92.4%,24 h内转化率和选择性维持在80%和82%以上。Abstract: ZSM-5 zeolite was modified by Fenton's reagent, FeSO4 and H2O2 aqueous solutions using impregnation method, respectively. All these catalysts were characterized by XRD, ICP-OES, N2 adsorption-desorption, NH3-TPD, Py-FTIR and evaluated in propene oligomerization process. The results demonstrated that the framework of the parent ZSM-5 was well preserved after modification with Fenton's reagent, FeSO4 or H2O2 solutions. However, the SiO2/Al2O3 ratios for all the modified ZSM-5 samples increased due to the dealumination. Furthermore, Fe was detected in Fenton-ZSM-5 while no Fe was observed for FeSO4-ZSM-5 catalyst. The BET surface areas and total pore volumes of three modified catalysts significantly increased compared with the original ZSM-5 sample. Among them, the BET surface area of the Fenton-ZSM-5 increased by 17.86%.The increase of mesopores was probably caused by the removal of the residual organic template in the catalysts due to the generation of·OH radicals by Fenton's reagent and H2O2. The Fenton-ZSM-5 catalyst formed new acid sites of Brønsted (B) and Lewis (L) with little change in the total calculated amount, which significantly changed the B/L ratio. Compared with the parent ZSM-5, the Fenton-ZSM-5 catalyst exhibited the best activity and stability for propene oligomerization reaction. The initial propene conversion and diesel selectivity were as high as 98.3% and 92.4%, respectively, and kept at >80% and >82% for about 24 h, respectively.
-
Key words:
- Fenton's reagent /
- ZSM-5 /
- FeSO4 /
- H2O2 /
- propene oligomerization
-
表 1 不同ZSM-5催化剂的织构性质和酸度定量
Table 1 Textural properties and acidity quantitative of different ZSM-5 catalysts
Catalyst Si/Ala
(molar ratio)Fe contenta SBET/
(m2·g-1)Pore volume v/(cm3·g-1) Acidity by strengthb Acidity by typec vmicro vmeso vtotal weak strong total Brønsted Lewis B/L ZSM-5 16.3 - 306.49 0.101 0.064 0.165 0.39 0.51 0.91 0.67 0.09 5.71 Fenton-ZSM-5 29.2 0.11 361.23 0.110 0.070 0.180 0.35 0.44 0.79 0.49 0.22 1.68 FeSO4-ZSM-5 33.5 - 359.99 0.115 0.058 0.175 0.36 0.46 0.82 0.44 0.20 1.62 H2O2-ZSM-5 37.6 - 359.30 0.111 0.071 0.183 0.35 0.44 0.80 0.63 0.05 8.89 note: SBET, BET surface area; vmicro, micropore volume determined by t-plot; vmeso, mesopore volume determined by vtotal-vmico a: measured by ICP;b: measured by NH3-TPD;c: measured by Py-FTIR 表 2 不同ZSM-5催化剂丙烯齐聚产物分布
Table 2 Products distribution for the propene oligomerization over the different ZSM-5 catalysts
Catalyst Carbon atom distribution /% C6 C9 C12 C15 C18+ HZSM-5 8.35 44.31 33.24 7.06 7.04 Fenton-ZSM-5 14.30 55.78 24.55 1.54 3.83 FeSO4-ZSM-5 15.32 53.88 24.82 1.59 4.39 H2O2-ZSM-5 14.55 55.09 24.38 2.19 3.78 -
[1] KIM Y T, CHADA J P, XU Z Y, PAGAN-TORRES J, ROSENFELD D C, WINNIFORD W L, SCHMIDT E, HUBER G W. Low-temperature oligomerization of 1-butene with H-ferrierite[J]. J Catal, 2015, 323:33-44. doi: 10.1016/j.jcat.2014.12.025 [2] ZHONG L S, YU F, AN Y L, ZHAO Y H, SUN Y H, LI Z J, LIN T J, LIN Y J, QI X Z, DAI Y Y, GU L, HU J S, JIN S F, SHEN Q, WANG H. Cobalt carbide nano prisms for direct production of lower olefins from syngas[J]. Nature, 2016, 538(7623):84-87. doi: 10.1038/nature19786 [3] CORMA A, MARTÍNEZ C, DOSKOCIL E. Designing MFI-based catalysts with improved catalyst life for C3 and C5 oligomerization to high-quality liquid fuels[J]. J Catal, 2013, 300:183-196. doi: 10.1016/j.jcat.2012.12.029 [4] WULFERS M J, LOBO R F. Assessment of mass transfer limitations in oligomerization of butene at high pressure on H-beta[J]. Appl Catal A:Gen, 2015, 505:394-401. doi: 10.1016/j.apcata.2015.08.016 [5] MARTÍNEZ C, CORMA A. Inorganic molecular sieves:Preparation, modification and industrial application in catalytic processes[J]. Coord Chem Rev, 2011, 255(13/14):1558-1580. http://cn.bing.com/academic/profile?id=3e53b825bb7de377adcdeb8990828ad3&encoded=0&v=paper_preview&mkt=zh-cn [6] MOON S, CHAE H, PARK M B. Oligomerization of light olefins over ZSM-5 and beta zeolite catalysts by modifying textural properties[J]. Appl Catal A:Gen, 2018, 553:15-23. doi: 10.1016/j.apcata.2018.01.015 [7] COETZEE J H, MASHAPA T N, PRINSLOO N M, RADEMAN J D. An improved solid phosphoric acid catalyst for alkene oligomerization in a Fischer-Tropsch refinery[J]. Appl Catal A:Gen, 2006, 308:204-209. doi: 10.1016/j.apcata.2006.04.023 [8] VAN GRIEKEN R, ESCOLA, ESCOLA J M, MORENO J, RODRIGUEZ R. Liquid phase oligomerization of 1-hexene over different mesoporous aluminosilicates (Al-MTS, Al-MCM-41 and Al-SBA-15) and micrometer/nanometer HZSM-5 zeolites[J]. Appl Catal A:Gen, 2006, 305(2):176-188. doi: 10.1016/j.apcata.2006.02.058 [9] PERATELLO S, MOLINARI M, BELLUSSI G, PEREGO C. Olefins oligomerization:Thermodynamics and kinetics over a mesoporous silica-alumina[J]. Catal Today, 1999, 52(2/3):271-277. http://cn.bing.com/academic/profile?id=8181fab3434cdc264d7aed26bed9d6ea&encoded=0&v=paper_preview&mkt=zh-cn [10] CATANI R, MANDREOLI M, ROSSINI S, VACCAR I. Mesoporous catalysts for the synthesis of clean diesel fuels by oligomerisation of olefins[J]. Catal Today, 2002, 75(1/4):125-131. http://cn.bing.com/academic/profile?id=f578c2cee0ff00fa7903fdee88a74d06&encoded=0&v=paper_preview&mkt=zh-cn [11] GALYA L G, OCCELLI M L, HSU J T, YOUNG D C. Propylene oligomerization over molecular sieves:Part II. 1H NMR and 13C NMR characterization of reaction products[J]. J Mol Catal, 1985, 32(3):391-403. doi: 10.1016/0304-5102(85)85093-8 [12] BELLUSSI G, MIZIA F, CALEMMA V, POLLESEL P, MILLINI R. Oligomerization of olefins from light cracking naphtha over zeolite-based catalyst for the production of high quality diesel fuel[J]. Microporous Mesoporous Mater, 2012, 164:127-134. doi: 10.1016/j.micromeso.2012.07.020 [13] CATHERINE S, CHEN H, ROBERT F, BRIDGE R. Shape-selective oligomerization of alkenes to near-linear hydrocarbons by zeolite catalysis[J]. J Catal, 1996, 161(2):687-693. doi: 10.1006/jcat.1996.0230 [14] QUANN R J, GREEN L A, TABAK S A, KRAMBECK F J. Chemistry of olefin oligomerization over ZSM-5 catalyst[J]. Chem Res, 1988, 27(4):565-570. http://cn.bing.com/academic/profile?id=593b9de42ce9a54f81cf6494fc7868aa&encoded=0&v=paper_preview&mkt=zh-cn [15] HUANG X, AIHEMAITIJIANG D, XIAO W D. Reaction pathway and kinetics of C3-C7 olefin transformation over high-silicon HZSM-5 zeolite at 400-490 degrees C[J]. Chem Eng J, 2015, 280:222-232. doi: 10.1016/j.cej.2015.05.124 [16] CHEN C J, RANGARAJAN S, HILL I M, BHAN A. Kinetics and Thermochemistry of C4-C6 Olefin Cracking on H-ZSM-5[J]. ACS Catal, 2014, 4(7):2319-2327. doi: 10.1021/cs500119n [17] SILVA A F, FERNANDES A, ANTUNES M M, NEVES P, ROCHA S M, RIBEIRO M F, PILLINGER M, RIBEIRO J, SILVA C M, VALENTE A A. Kinetics and thermochemistry of C4-C6 olefin cracking on H-ZSM-5[J]. Fuel, 2017, 209(7):371-382. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=42d93344b97b319c767d3e2a98758e88 [18] HULEA V, FAJULA F J. Ni-exchanged AlMCM-41-An efficient bifunctional catalyst for ethylene oligomerization[J]. Catal 2004, 225(1):213-222. doi: 10.1016/j.jcat.2004.04.018 [19] MENG F J, WANG Y Q, WANG S H. Methanol to gasoline over zeolite ZSM-5:Improved catalyst performance by treatment with HF[J]. RSC Adv, 2016, 6(63):58586-58593. doi: 10.1039/C6RA14513B [20] LI X M, HAN D Z, WANG H, LIU G B, WANG B, LI Z, WU J H. Propene oligomerization to high-quality liquid fuels over Ni/HZSM-5[J]. Fuel, 2015, 144:9-14. doi: 10.1016/j.fuel.2014.12.005 [21] FENG G D, CHENG P, YAN W F, BORONAT M, LI X, SU J H, WANG J, LI Y, CORMA A, XU R, YU J H. Accelerated crystallization of zeolites via hydroxyl free radicals[J]. Science, 2016, 351(6278):1188-1191. doi: 10.1126/science.aaf1559 [22] KONECNY R. Reactivity of hydroxyl radicals on hydroxylated quartz surface. 1. Cluster model calculations[J]. J Phys Chem B, 2001, 105(26):6221-6226. doi: 10.1021/jp010752v [23] LI C, DU J, WANG H, LI X, ZHU S, LIU G, WU J H. A hemicellulose modified HZSM-5 and their application in the light olefins oligomerization to high-quality liquid fuels reaction[J]. Catal Commun, 2017, 102:89-92. doi: 10.1016/j.catcom.2017.08.032 [24] 王明明, 姚志龙, 赵如松. H2O2处理对HZSM-5分子筛结构及催化环己烯水合性能的影响[J].工业催化, 2011, 19(9):39-44. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gych201109008WANG Ming-ming, YAO Zhi-long, ZHAO Ru-song. Effects of H2O2 treatment on structure and catalytic performance of HZSM-5 molecular sieves for hydration of cyclohexene[J]. Ind Catal, 2011, 19(9):39-44. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gych201109008 [25] CABRERA M L, KAPTEIJN F, MOULIJN J A. One-pot catalyst preparation:Combined detemplating and Fe ionexchange of BEA through Fenton's chemistry[J]. Chem Commun, 2005, 16:2178-2180. [26] WU G, HEI F, GUAN N, LI L. Oxidative dehydrogenation of propane with nitrous oxide over Fe-MFI prepared by ion-exchange:Effect of acid post-treatments[J]. Catal Sci Technol, 2013, 3(5):1333-1342. doi: 10.1039/c3cy20782j [27] LI J Q, HAN D Z, HE T, LIU G B, ZI Z Y, WANG Z J, WU J H. Nanocrystal H[Fe, Al] ZSM-5 zeolites with different silica-alumina composition for conversion of dimethyl ether to gasoline[J]. Fuel Processing Technol, 2019, 191:104-110. doi: 10.1016/j.fuproc.2019.03.029 [28] LIU Z, WU D, REN S, CHEN X, QIU M, LIU G, ZENG G, SUN Y. Facile one-pot solvent-free synthesis of hierarchical ZSM-5 for methanol to gasoline conversion[J]. RSC Adv, 2016, 6(19):15816-15820. doi: 10.1039/C6RA00247A [29] WANG X, ZHANG J, ZHANG T, XIAO H, SONG F, HAN Y, TAN Y. Mesoporous ZnZSM-5 zeolites synthesized by one-step desilication and reassembly:A durable catalyst for methanol aromatization[J]. RSC Adv, 2016, 6(28):23428-23437. doi: 10.1039/C6RA03511F [30] XU T, ZHANG Q, SONG H, WANG Y. Fluoride-treated H-ZSM-5 as a highly selective and stable catalyst for the production of propylene from methyl halides[J]. J Catal, 2012, 295:232-241. doi: 10.1016/j.jcat.2012.08.014 [31] NKOSI B, NG F T T, REMPEL G L. The oligomerization of butenes with partially alkali exchanged NiNaY zeolite catalysts[J]. Appl Catal A:Gen, 1997, 158(12):225-241. http://cn.bing.com/academic/profile?id=2cd463944a5fa00aaed36c9ad6141da9&encoded=0&v=paper_preview&mkt=zh-cn -





 下载:
下载: