Preparation of layered K/Mg-Fe-Al catalysts and its catalytic performances in CO hydrogenation
-
摘要: 采用共沉淀法制备了系列不同Mg/Fe/Al配比MgFeAl-HTLcs前驱体,经焙烧、浸渍K改性、二次焙烧后用于CO加氢反应。采用N2吸附-脱附、SEM、TG、XRD、H2-TPR、XPS等手段对催化剂进行了表征。结果表明,共沉淀法制备的不同配比MgFeAl-HTLcs类水滑石前躯体均具有典型层状结构;焙烧后生成MgO、Fe2O3以及少量MgFeAlO4物相,三组元间相互作用增强,反应后以MgCO3和Fe3O4物相为主,同时出现较弱的Fe5C2相;K改性后发生结构重构,热稳定性增强,且随Al含量增加,比表面积显著单调下降;与K/Mg-Fe相比,K/Mg-Fe-Al样品中Fe2O3到Fe3O4的还原受到抑制;二次焙烧后,反应前表面相对富Fe,反应后表面富K。在CO加氢反应中,K/Mg-Fe-Al系列催化剂均表现出较高的反应活性以及烯烃选择性,随Fe/Al配比相对增加,C5+含量呈降低趋势,O/P值增加;与K/1.5Mg-0.67Fe相比,K/1.5Mg-0.67Fe-0.33Al催化剂C5+含量由22.17%降至10.90%,C2-4=含量由40.98%提高至47.28%。
-
关键词:
- 费托合成 /
- K/Mg-Fe-Al催化剂 /
- CO加氢 /
- 低碳烯烃
Abstract: A series of K promoted K/MgFeAl-HTLcs catalysts with different Mg/Fe/Al molar ratios were prepared by means of coprecipitation and impregnation method for direct synthesis of light olefins from CO hydrogenation. The samples were characterized by XRD, N2 adsorption-desorption, SEM, TG, H2-TPR and XPS measurements. The results show that MgFeAl-HTLcs catalyst precursors has typical layered structure. MgO, Fe2O3 and small amount of MgFeAlO4 are formed after calcination. MgCO3 and Fe3O4 could be observed after reaction, and a little Fe5C2 iron carbide with broad and weak peaks appear simultaneously. Thermal stability of K/MgFeAl-HTLcs is improved due to recovery of hydrotalcite-like structure after K promotion. With increase of Al content, specific surface area of the precursors decreases monotonically after structure reconstruction. Reduction of Fe2O3 to Fe3O4 is inhibited with addition of Al, compared with K/Mg-Fe sample. Fe enrichment before reaction and K enrichment after reaction are observed on secondary calcination samples. During CO hydrogenation, the prepared samples show high activity and C2-4= selectivity with low C5+ weight fraction. C5+ hydrocarbons decrease and olefin selectivity increases with increasing Fe/Al molar ratio. The C5+ decreases from 22.17% to 10.90%, and C2-4= weight content increases from 40.98% to 47.28% on K/1.5Mg-0.67Fe-0.33Al sample compared with that of K/1.5Mg-0.67Fe sample.-
Key words:
- Fischer-Tropsch synthesis /
- K/Mg-Fe-Al catalyst /
- CO hydrogenation /
- light olefins
-
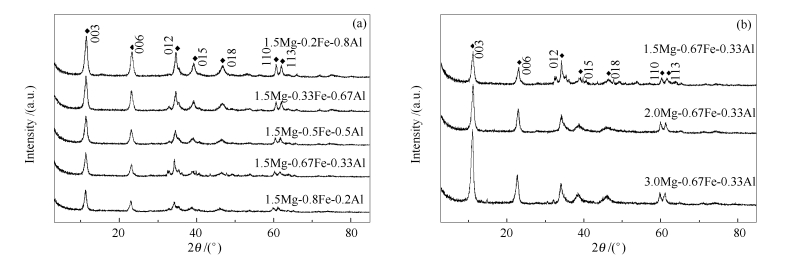
表 1 催化剂的织构性质
Table 1 Textural properties of the catalysts
Catalyst BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average pore size d/nm 1.5Mg-0.67Fe-0.33Al 83.06 0.60 28.39 K/1.5Mg-0.2Fe-0.8Al 131.09 0.23 7.11 K/1.5Mg-0.2Fe-0.8Al-Ha 11.00 0.05 18.91 K/1.5Mg-0.33Fe-0.67Al 167.58 0.25 5.96 K/1.5Mg-0.33Fe-0.67Al-Ha 15.74 0.05 13.07 K/1.5Mg-0.5Fe-0.5Al 116.85 0.21 7.26 K/1.5Mg-0.67Fe-0.33Al 49.84 0.16 12.50 K/1.5Mg-0.67Fe-0.33Al-Ha 33.20 0.08 9.78 K/1.5Mg-0.8Fe-0.2Al 64.56 0.20 12.38 K/1.5Mg-0.8Fe-0.2Al-Ha 17.32 0.06 13.19 K/1.5Mg-0.67Fe 103.15 0.24 9.37 K/1.5Mg-0.67Fe-Ha 50.95 0.10 7.86 K/2.0Mg-0.67Fe-0.33Al 83.14 0.19 9.28 K/3.0Mg-0.67Fe-0.33Al 84.77 0.19 9.06 a: K promoted samples without calcination 表 2 不同条件下催化剂的表面组成
Table 2 Surface composition of different samples
Sample Surface atom content wartom/%a Mg Fe Al K O C Mg/Fe Fe/Al Fe/K K/1.5Mg-0.2Fe-0.8Alb 17.20 0.84 10.33 - 53.94 17.69 20.48 0.08 - K/1.5Mg-0.2Fe-0.8Al-Hb 17.04 1.18 8.80 0.14 55.25 17.60 14.44 0.13 8.43 K/1.5Mg-0.67Fe-0.33Alb 17.87 3.66 5.90 0.29 53.03 19.26 4.88 0.62 12.62 K/1.5Mg-0.67Fe-0.33Al-Hb 19.60 4.45 4.18 0.31 51.94 19.53 4.40 1.06 14.35 K/1.5Mg-0.8Fe-0.2Alb 18.96 6.57 5.14 0.38 53.95 15.00 2.89 1.28 17.29 K/1.5Mg-0.67Feb 21.89 9.00 - 0.26 52.31 16.55 2.43 - 34.62 K/2.0Mg-0.67Fe-0.33Alb 21.60 4.81 5.88 0.25 54.20 13.27 4.49 0.82 19.24 K/1.5Mg-0.2Fe-0.8Alc 9.73 0.63 11.95 - 40.49 37.21 15.44 0.05 - K/1.5Mg-0.2Fe-0.8Al-Hc 8.78 0.45 7.56 - 31.47 51.75 19.51 0.06 - K/1.5Mg-0.67Fe-0.33Alc 1.16 0.36 1.63 0.22 17.35 79.29 3.22 0.22 1.64 K/1.5Mg-0.67Fe-0.33Al-Hc 14.84 3.00 5.95 0.17 43.70 32.35 4.95 0.50 17.65 K/1.5Mg-0.8Fe-0.2Alc 1.84 0.77 1.96 0.48 22.98 71.97 2.39 0.39 1.60 K/1.5Mg-0.67Fec 1.84 0.56 - 0.62 18.44 78.54 3.29 - 0.90 K/2.0Mg-0.67Fe-0.33Alc 3.56 0.81 2.43 0.15 23.14 78.54 4.40 0.33 5.40 a: calculated from the peak area of XPS spectra; b: fresh samples; c: used samples 表 3 催化剂的CO加氢反应性能
Table 3 Catalytic performance of the catalysts
Catalyst CO Conv. x/% Selectivity s/% Product w/% O/P CH4 CO2 CH4 C2-4= C2-40 C5+ K/1.5Mg-0.2Fe-0.8Al 87.78 15.71 24.36 29.18 39.51 18.63 12.68 2.12 K/1.5Mg-0.33Fe-0.67Al 82.60 20.22 16.55 30.62 35.92 19.30 14.15 1.86 K/1.5Mg-0.5Fe-0.5Al 77.86 20.76 14.73 29.48 41.79 15.88 12.85 2.63 K/1.5Mg-0.67Fe-0.33Al 69.47 22.41 10.06 30.48 47.28 11.35 10.90 4.16 K/1.5Mg-0.67Fe-0.33Al-H 96.70 12.53 18.18 30.57 38.61 18.43 12.39 2.10 K/1.5Mg-0.8Fe-0.2Al 73.09 22.57 15.94 32.88 46.18 10.73 10.21 4.30 K/1.5Mg-0.8Fe-0.2Al-H 74.97 23.50 14.99 33.22 44.40 11.59 10.79 3.83 K/2.0Mg-0.67Fe-0.33Al 67.54 28.14 8.11 35.52 42.01 12.79 9.68 3.29 K/3.0Mg-0.67Fe-0.33Al 61.13 29.67 5.90 35.30 41.17 13.52 10.01 3.05 K/1.5Mg-0.67Fe 81.45 22.04 10.11 27.00 40.98 9.86 22.17 4.16 K/1.5Mg-0.67Fe-H 93.39 12.64 32.06 20.86 35.87 10.50 32.77 3.41 reaction conditions: H2/CO=2, GHSV=1 000 h-1, t=320 ℃, p=1.5 MPa; -
[1] TORRES GALVIS H M, BITTER J H, KHARE C B, RUITENBEEK M, IULIAN DUGULAN A, DE JONG K P. Supported iron nanoparticles as catalysts for sustainable production of lower olefins[J]. Science, 2012, 335(6070):835-838. doi: 10.1126/science.1215614 [2] JIAO F, LI J J, PAN X L, XIAO J P, LI H B, MA H, WEI M M, PAN Y, ZHOU Z Y, LI M R, MIAO S, LI J, ZHU Y F, XIAO D, HE T, YANG J H, QI F, FU Q, BAO X H. Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277):1065-1068. doi: 10.1126/science.aaf1835 [3] CHENG K, GU B, LIU X L, KANG J C, ZHANG Q H, WANG Y. Direct and highly selective conversion of synthesis gas into lower olefins:design of a bifunctional catalyst combining methanol synthesis and carbon-carbon coupling[J]. Angew Chem Int Ed, 2016, 55(15):4725-4728. doi: 10.1002/anie.201601208 [4] ZHONG L S, YU F, AN Y L, ZHAO Y H, SUN Y H, LI Z J, LIN T J, LIN Y J, QI X Z, DAI Y Y, GU L, HU J S, JIN S F, SHEN Q, WANG H. Cobalt carbide nanoprisms for direct production of lower olefins from syngas[J]. Nature, 2016, 538(7623):84-87. doi: 10.1038/nature19786 [5] ZHAI P, XU C, GAO R, LIU X, LI M Z, LI W Z, FU X P, JIA C J, XIE J L, ZHAO M, WANG X P, LI Y W, ZHANG Q W, WEN X D, MA D. Highly tunable selectivity for syngas-derived alkenes over zinc and sodium-modulated Fe5C2 catalyst[J]. Angew Chem Int Ed, 2016, 55(34):9902-9907. doi: 10.1002/anie.201603556 [6] 于飞, 李正甲, 安芸蕾, 高鹏, 钟良枢, 孙予罕. 合成气催化转化直接制备低碳烯烃研究进展[J]. 燃料化学学报, 2016, 44(7): 801-814. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201607005&dbname=CJFD&dbcode=CJFQYU Fei, LI Zheng-jia, AN Yun-lei, ZHONG Liang-shu, SUN Yu-han. Research progress in the direct conversion of syngas to lower olefins. J Fuel Chem Technol, 2016, 44(7):801-814. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201607005&dbname=CJFD&dbcode=CJFQ [7] CAVANI F, TRIFIRÒF, VACCARI A. Hydrotalcite-type anionic clays:Preparation, properties and applications[J]. Catal Today, 1991, 11(2):173-301. doi: 10.1016/0920-5861(91)80068-K [8] 高鹏, 李枫, 赵宁, 王慧, 魏伟, 孙予罕.以类水滑石为前驱体的Cu/Zn/Al/(Zr)/(Y)催化剂制备及其催化CO2加氢合成甲醇的性能[J].物理化学学报, 2014, 30(6):1155-1162. doi: 10.3866/PKU.WHXB201401252GAO Peng, LI Feng, ZHAO Ning, WANG Hui, WEI Wei, SUN Yu-han. Preparation of Cu/Zn/Al/(Zr)/(Y) catalysts from hydrotalcite-like precursors and their catalytic performance for the hydrogenation of CO2 to methanol[J]. Acta Phys-Chim Sin, 2014, 30(6):1155-1162. doi: 10.3866/PKU.WHXB201401252 [9] FRONZO A D, PIROLA C, COMAZZI A, GALLI F, BIANCHI C L, MICHELE A D, VIVANI R, NOCCHETTI M, BASTIANINI M, BOFFITO D C. Co-based hydrotalcites as new catalysts for the Fischer-Tropsch synthesis process[J]. Fuel, 2014, 119(3):62-69. http://www.sciencedirect.com/science/article/pii/S0016236113010600 [10] 谢鲜梅. 类水滑石化合物的制备、性能及应用研究[D]. 太原: 太原理工大学, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10112-2008018215.htmXIE Xian-mei. Study on the preparation, performances and application of hydrotalcite-like-compounds[D]. Taiyuan:Taiyuan University of Technology, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10112-2008018215.htm [11] 马丽萍, 张建利, 马清祥, 范素兵, 赵天生. K/MgFeZn-HTLcs催化CO加氢制低碳烯烃性能研究[J].燃料化学学报, 2016, 44(4):449-456. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18812.shtmlMA Li-ping, ZHANG Jian-li, MA Qing-xiang, FAN Su-bing, ZHAO Tian-sheng. Direct synthesis of light olefins from CO hydrogenation over K/MgFeZn-HTLcs catalysts[J]. J Fuel Chem Technol, 2016, 44(4):449-456. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18812.shtml [12] VISCONTI C G, LIETTI L, TRONCONI E, FORZATTI P, ZENNARO R, FINOCCHIO E. Fischer-Tropsch synthesis on a Co/Al2O3 catalyst with CO2 containing syngas[J], Appl Catal A:Gen, 2009, 355(1/2):61-68. http://www.sciencedirect.com/science/article/pii/S0926860X08007308 [13] PARK S J, BAE J W, OH J H, CHARY K V R, SAI PRASAD P S, JUN K W, RHEE Y W. Influence of bimodal pore size distribution of Ru/Co/ZrO2-Al2O3 during Fischer-Tropsch synthesis in fixed-bed and slurry reactor[J]. J Mol Catal A:Chem, 2009, 298(1):81-87. http://www.sciencedirect.com/science/article/pii/S1381116908004639 [14] TOSHIYUKI HIBINO, YASUMASA Y, KATSUNORI K, ATSUMU T. Decarbonation behavior of Mg-A1-CO3 hydrotalcite-like compounds during heat treatment[J]. Clay Clay Miner, 1995, 43(4):427-432. doi: 10.1346/CCMN [15] VALENTE J S, PRINCE J, MAUBERT A M, LARTUNDO-ROJAS L, ANGEL P D, FERRAT G, HERNANDEZ J G, LOPEZ-SALINAS E. Physicochemical study of nanocapsular layered double hydroxides evolution[J]. J Phys Chem C, 2009, 113(14):5547-5555. doi: 10.1021/jp810293y [16] ZHAO M Q, ZHANG Q, ZHANG W, HUANG J Q, ZHANG Y H, SU D S, WEI F. Embedded high density metal nanoparticles with extraordinary thermal stability derived from guest-host mediated layered double hydroxides[J]. J Am Chem Soc, 2010, 132(42):14739-14741. doi: 10.1021/ja106421g [17] JOZWIAK W K, KACZMAREK E, MANIECKI T P, IGNACZAK W, MANIUKIEWICZ W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres[J]. Appl Catal A:Gen, 2007, 326(1):17-27. doi: 10.1016/j.apcata.2007.03.021 [18] LI S, LI A, KRISHNAMOORTHY S, IGLESIA E. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts[J]. Catal Lett, 2001, 77(4):197-205. doi: 10.1023/A:1013284217689 [19] SUO H, WANG S, ZHANG C, XU J, WU B, YANG Y, XIANG H, LI Y. Chemical and structural effects of silica in iron-based Fischer-Tropsch synthesis catalysts[J]. J Catal, 2012, 286(2):111-123. http://www.sciencedirect.com/science/article/pii/S0021951711003599 [20] GAO X, SHEN J, HSIA Y, CHEN Y. Reduction of supported iron oxide studied by temperature-programmed reduction combined with mössbauer spectroscopy and X-ray diffraction[J]. J Chem Soc Faraday Trans, 1993, 89(7):1079-1084. doi: 10.1039/FT9938901079 [21] CHEN K, FAN Y, HU Z, YAN Q. Carbon monoxide hydrogenation on Fe2O3/ZrO2 catalysts[J]. Catal Lett, 1996, 36(3/4):139-144. doi: 10.1007/BF00807610 [22] QIN S D, ZHANG C H, XU J, YANG Y, XIANG H W, LI Y W. Fe-Mo interactions and their influence on Fischer-Tropsch synthesis performance[J]. Appl Catal A:Gen, 2011, 392(1/2):118-126. http://www.sciencedirect.com/science/article/pii/S0926860X10007386 [23] HUO C F, WU B S, GAO P, YANG Y, LI Y W, JIAO H J. The mechanism of potassium promoter:Enhancing the stability of active surfaces[J]. Angew Chem Int Edit, 2011, 50(32):7403-7406. doi: 10.1002/anie.v50.32 -





 下载:
下载: