A comparison of Al2O3 and SiO2 supported Ni-based catalysts in their performance for the dry reforming of methane
-
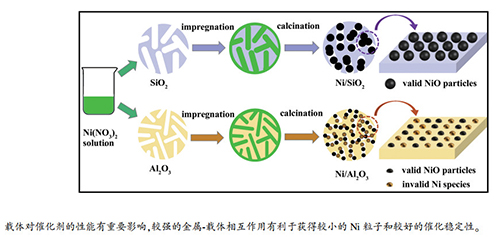
摘要: CH4与CO2干重整反应对于环境保护和天然气资源的合理利用具有重要意义。SiO2和Al2O3是适用于甲烷干重整反应的两种典型的催化剂载体。为了阐明这两种载体对催化剂性能的影响,本研究采用等体积浸渍法制备了Ni/Al2O3和Ni/SiO2催化剂,并利用BET、TEM、H2-TPR、XRD、TG和Raman等技术对还原和反应后的催化剂进行了表征。结果表明,由于载体的性质不同,Ni基催化剂在甲烷干重整中的催化性能也不同。Ni/SiO2催化剂的初始活性较高,但由于其金属-载体相互作用较弱,催化稳定性较差,在800℃下反应15h其催化活性急剧下降;较弱的金属-载体相互作用使得Ni/SiO2催化剂上的Ni颗粒较大,有利于积炭前驱物种的生成,导致催化剂快速失活。而对于Ni/Al2O3催化剂,金属-载体相互作用较强,Ni颗粒较小,但由于Ni与Al2O3生成了NiAlxOy物种,有效活性位减少,其催化活性相对较低,但催化稳定性较好,干重整反应进行50h其活性保持稳定;Ni与Al2O3之间较强的相互作用有利于形成小且稳定的Ni粒子,能减少积炭,因而具有优异的催化稳定性。Abstract: Dry reforming of methane (DRM) with CO2 is of great significance in the environmental protection and the utilization of natural gas. SiO2 and Al2O3 are two typical catalyst supports used in DRM. To elucidate the effect of these two supports on the catalytic performance, in this work, Ni/SiO2 and Ni/Al2O3 catalysts are prepared by the incipient wetness method and characterized by BET, TEM, H2-TPR, XRD, TG and Raman technologies. The results indicate that the performance of Ni-based catalyst is closely related to the properties of support and the Ni/SiO2 and Ni/Al2O3 catalysts are rather different in their DRM performance. Ni/SiO2 catalyst exhibits higher initial activity but poor stability; its catalytic activity decreases rapidly in 15 h for DRM at 800℃. Because of the weak metal-support interaction, Ni species on the Ni/SiO2 catalyst is present as large Ni particles, which may promote the formation of coke precursors, viz., the multi-carbon Cn species, leading to the fast carbonaceous deposition and catalyst deactivation. In contrast, the Ni/Al2O3 catalyst displays a lower activity but a much higher stability; its activity in DRM keeps stable in 50 h. Although Ni particles in the Ni/Al2O3 catalyst is much smaller, the strong metal-support interaction promotes the formation of NiAlxOy species during the catalyst preparation process, which may lead to a decrease in the content of active Ni species and give the Ni/Al2O3 catalyst a relatively low catalytic activity in DRM; however, the strong metal-support interaction between Ni and Al2O3 is also of benefit to the formation and stabilization of small Ni particles, which can alleviate the carbanceous deposition and afford the Ni/Al2O3 catalyst a better stability.
-
Key words:
- methane /
- dry reforming /
- nickel-based catalyst /
- structure-activity relationship /
- support effect /
- Ni/Al2O3 /
- Ni/SiO2
-
Table 1 Textural properties of various catalysts
Catalyst ABET/
(m2·g-1)vpore/
(cm3·g-1)Pore diameter
d/nmvpore/ABET
(10-9 m)Reduced Ni/SiO2 162.6 0.99 15.5 6.1 Reduced Ni/Al2O3 159.3 0.34 6.2 2.1 Spent Ni/SiO2 89.9 0.45 17.5 - Spent Ni/Al2O3 152.1 0.35 7.4 - -
[1] CABALLERO A, PEREZ P J. Methane as raw material in synthetic chemistry:The final frontier[J]. Chem Soc Rev, 2013, 42(23):8809-8820. doi: 10.1039/c3cs60120j [2] OLSBYE U. Single-pass catalytic conversion of syngas into olefins via methanol[J]. Angew Chem Int Ed, 2016, 55(26):7294-7295. doi: 10.1002/anie.201603064 [3] VENVIK H J, YANG J. Catalysis in microstructured reactors:Short review on small-scale syngas production and further conversion into methanol, DME and Fischer-Tropsch products[J]. Catal Today, 2017, 285:135-146. doi: 10.1016/j.cattod.2017.02.014 [4] ALI K A, ABDULLAH A Z, MOHAMED A R. Recent development in catalytic technologies for methanol synthesis from renewable sources:A critical review[J]. Renewable Sustainable Energy Rev, 2015, 44(32):508-518. https://www.sciencedirect.com/science/article/pii/S1364032115000209 [5] HU J, YU F, LU Y. Application of Fischer-Tropsch synthesis in biomass to liquid conversion[J]. Catalysts, 2012, 2(2):303-326. doi: 10.3390/catal2020303 [6] USMAN M, DAUD W M A W, ABBAS H F. Application of Fischer-Tropsch synthesis in biomass to liquid conversion[J]. Renewable Sustainable Energy Rev, 2015, 45:710-744. doi: 10.1016/j.rser.2015.02.026 [7] SHAH Y T, GARDNER T H. Dry reforming of hydrocarbon feedstocks[J]. Catal Rev, 2014, 56(4):476-536. doi: 10.1080/01614940.2014.946848 [8] MURAZA O, GALADIMA A. A review on coke management during dry reforming of methane[J]. Int J Energy Res, 2015, 39(9):1196-1216. doi: 10.1002/er.v39.9 [9] ABDULLAH B, GHANI N A A, VO D V N. Recent advances in dry reforming of methane over Ni-based catalysts[J]. J Clean Prod, 2017, 162:170-185. doi: 10.1016/j.jclepro.2017.05.176 [10] ZHANG X, YANG C, ZHANG Y, XU Y, SHANG S, YIN Y. Ni-Co catalyst derived from layered double hydroxides for dry reforming of methane[J]. Int J Hydrogen Energy, 2015, 40(46):16115-16126. doi: 10.1016/j.ijhydene.2015.09.150 [11] DAS S, ASHOK J, BIAN Z, DEWANGAN N, WAI M H, DU Y, BORGNA A, HIDAJAT K, KAWI S. Silica-ceria sandwiched ni core-shell catalyst for low temperature dry reforming of biogas:Coke resistance and mechanistic insights[J]. Appl Catal B:Environ, 2018, 230:220-236. doi: 10.1016/j.apcatb.2018.02.041 [12] DAI C, ZHANG S, ZHANG A, SONG C, SHI C, GUO X. Hollow zeolite encapsulated Ni-Pt bimetals for sintering and coking resistant dry reforming of methane[J]. J Mater Chem A, 2015, 3(32):16461-16468. doi: 10.1039/C5TA03565A [13] WANG C Z, SI L J, LI H, WEN X, SUN N N, ZHAO N, WEI W, SUN Y H. Template-free one-pot synthesis of mesoporous Ni-CaO-ZrO2 catalyst and its application in CH4-CO2 reforming[J]. J Fuel Chem Technol, 2013, 41(10):1204-1209. doi: 10.1016/S1872-5813(13)60049-3 [14] WANG C Z, SUN N N, ZHAO N, WEI W, ZHAO Y X. Template-free preparation of bimetallic mesoporous Ni-Co-CaO-ZrO2 catalysts and their synergetic effect in dry reforming of methane[J]. Catal Today, 2017, 281:268-275. doi: 10.1016/j.cattod.2016.03.026 [15] WANG C Z, SUN N N, ZHAO N, WEI W, SUN Y H, SUN C G, LIU H, SNAPE C E. Coking and deactivation of a mesoporous Ni-CaO-ZrO2 catalyst in dry reforming of methane:A study under different feeding compositions[J]. Fuel, 2015, 143:527-535. doi: 10.1016/j.fuel.2014.11.097 [16] HUANG X, JI C, WANG C Z, XIAO F K, ZHAO N, SUN N N, WEI W, SUN Y H. Ordered mesoporous CoO-NiO-Al2O3 bimetallic catalysts with dual confinement effects for CO2 reforming of CH4[J]. Catal Today, 2017, 281:241-249. doi: 10.1016/j.cattod.2016.02.064 [17] HUANG X, XUE G X, WANG C Z, ZHAO N, SUN N N, WEI W, SUN Y H. Highly stable mesoporous NiO-Y2O3-Al2O3 catalysts for CO2 reforming of methane:Effect of Ni embedding and Y2O3 promotion[J]. Catal Sci Technol, 2016, 6:449-459. doi: 10.1039/C5CY01171J [18] WANG C Z, QIU Y, ZHANG X M, ZHANG Y, SUN N N, ZHAO Y X. Geometric art of a Ni@silica nano-capsule catalyst with superb methane dry reforming stability:Enhanced confinement effect over nickel site anchoring inside capsule shell with appropriate inner cavity[J]. Catal Sci Technol, 2018, 8:4877-4890. doi: 10.1039/C8CY01158C [19] YANG W W, LIU H M, LI Y M, ZHANG J, WU H, HE D H. Properties of yolk-shell structured Ni@SiO2 nanocatalyst and its catalytic performance in carbon dioxide reforming of methane to syngas[J]. Catal Today, 2016, 259:438-445. doi: 10.1016/j.cattod.2015.04.012 [20] DAS S, ASHOK J, BIAN Z, DEWANGAN N, WAI M H, DU Y, BORGNA A, HIDAJAT K, KAWI S. Silica-ceria sandwiched ni core-shell catalyst for low temperature dry reforming of biogas:Coke resistance and mechanistic insights[J]. Appl Catal B:Environ, 2018, 230:220-236. https://www.sciencedirect.com/science/article/pii/S0926337318301620 [21] WANG Y S, FANG Q, SHEN W H, ZHU Z Q, FANG Y J. (Ni/MgAl2O4)@SiO2 core-shell catalyst with high coke-resistance for the dry reforming of methane[J]. React Kinet Mech Catal, 2018, 125(1):127-139. doi: 10.1007/s11144-018-1404-2 [22] PU J L, LUO Y, WANG N N, BAO H X, WANG X H, QIAN E W. Ceria-promoted Ni@Al2O3 core-shell catalyst for steam reforming of acetic acid with enhanced activity and coke resistance[J]. Int J Hydrogen Energy, 2018, 43(6):3142-3153. doi: 10.1016/j.ijhydene.2017.12.136 [23] CHAI Y, FU Y, FENG H, KONG W, YUAN C, PAN B, ZHANG J, SUN Y. Ni-based perovskite catalyst with a bimodal size distribution of Ni Particles for dry reforming of methane[J]. ChemCatChem, 2018, 9(10):2078-2086. [24] BAUDOUIN D, RODEMERCK U, KRUMEICH F, MALLMANN A D, SZETO K C, MÉNARD H, YEYRE L, CANDY J P, WEBB P B, THIEULEUX C, COPÉRET C. Particle size effect in the low temperature reforming of methane by carbon dioxide on silica-supported Ni nanoparticles[J]. J Catal, 2013, 297(1):27-34. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5a3a8aeef1975d7b6249cc33c7a11629 [25] GONZALEZDELACRUZ V M, PEREÑIGUEZ R, TERNERO F, HOLGADO J P, CABALLERO A. Modifying the size of nickel metallic particles by H2/CO treatment in Ni/ZrO2 methane dry reforming catalysts[J]. ACS Catal, 2013, 1(2):82-88. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8ed03165aba6565ba700abd704571e19 [26] DAOURA O, KAYDOUH M N, EI-HASSAN N, MASSIANI P, LAUNAY F, BOUTROS M. Mesocellular silica foam-based Ni catalysts for dry reforming of CH4 by CO2[J]. J CO2 Util, 2018, 24:112-119. doi: 10.1016/j.jcou.2017.12.010 [27] KIM W Y, LEE Y H, PARK H, CHOI Y H, LEE M H, LEE J S. Coke tolerance of Ni/Al2O3 nanosheet catalyst for dry reforming of methane[J]. Catal Sci Technol, 2016, 6(7):2060-2064. doi: 10.1039/C6CY00017G [28] AL-FATESH A S, ARAFAT Y, ATIA H, IBRAHIM A A, HA Q L M, SCHNEIDER M, M-POHL M, FAKEEHS A H. CO2 reforming of methane to produce syngas over Co-Ni/SBA-15 catalyst:Effect of support modifiers (Mg, La and Sc) on catalytic stability[J]. J CO2 Util, 2017, 21:395-404. doi: 10.1016/j.jcou.2017.08.001 [29] MO W, MA F, LIU Y, LIU J, ZHONG M, NULAHONG A. Preparation of porous Al2O3 by template method and its application in Ni-based catalyst for CH4/CO2 reforming to produce syngas[J]. Int J Hydrogen Energy, 2015, 40(46):16147-16158. doi: 10.1016/j.ijhydene.2015.09.149 [30] JEONG M G, KIM S Y, KIM D H, HAN S W, KIM I H, LEE M, HWANG Y K, KIM Y D. High-performing and durable MgO/Ni catalysts via atomic layer deposition for CO2 reforming of methane (CRM)[J]. Appl Catal A:Gen, 2016, 515:45-50. doi: 10.1016/j.apcata.2016.01.032 [31] ZENG S, ZHANG X, FU X, ZHANG L, SU H, PAN H. Co/CexZr1-xO2 solid-solution catalysts with cubic fluorite structure for carbon dioxide reforming of methane[J]. Appl Catal B:Environ, 2013, 136-137:308-316. doi: 10.1016/j.apcatb.2013.02.019 [32] SAHA B, KHAN A, IBRAHIM H, IDEM R. Evaluating the performance of non-precious metal based catalysts for sulfur-tolerance during the dry reforming of biogas[J]. Fuel, 2014, 120(1):202-217. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d0b2771c82d3e629eec58145fc3a1535 [33] CHEN X, JIANG J, TIAN S, LI K. Biogas dry reforming for syngas production:Catalytic performance of nickel supported on waste-derived SiO2[J]. Catal Sci Technol, 2015, 5(2):860-868. doi: 10.1039/C4CY01126K [34] SUN G B, HIDAJAT K, WU X S, KAWI S. A crucial role of surface oxygen mobility on nanocrystalline Y2O3 support for oxidative steam reforming of ethanol to hydrogen over Ni/Y2O3 catalysts[J]. Appl Catal B:Environ, 2008, 81(3):303-312. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=71219d4aa161b5776871cae263b1ce46 [35] OEMAR U, KATHIRASER Y, MO L, HO X K, KAWI S. CO2 reforming of methane over highly active La-promoted Ni supported on SBA-15 catalysts:Mechanism and kinetic modelling[J]. Catal Sci Technol, 2016, 6(4):1173-1186. doi: 10.1039/C5CY00906E [36] GOULA M A, CHARISIOU N D, PAPAGERIDIS K N, DELIMITIS A, PACHATOURIDOU E, ILIOPOULOU E F. Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction:Influence of the synthesis method[J]. Int J Hydrogen Energy, 2015, 40(30):9183-9200. doi: 10.1016/j.ijhydene.2015.05.129 [37] LI Z, KAWI S. Multi-Ni@Ni phyllosilicate hollow sphere for CO2 reforming of CH4:Influence of Ni precursors on structure, sintering and carbon resistance[J]. Catal Sci Technol, 2018, 8(7):1915-1922. doi: 10.1039/C8CY00024G [38] ZHANG C, YUE H, HUANG Z, LI S, WU G, MA X, GONG J. Hydrogen production via steam reforming of ethanol on phyllosilicate-derived Ni/SiO2:Enhanced metal-support interaction and catalytic stability[J]. ACS Sustainable Chem Eng, 2013, 1(1):161-173. doi: 10.1021/sc300081q [39] LIU C J, YE J Y, JIANG J J, PAN Y X. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane[J]. ChemCatChem, 2011, 3(3):529-541. doi: 10.1002/cctc.v3.3 [40] NUMAGUCHI T, EIDA H, SHOJI K. Reduction of NiAl2O4containing catalysts for steam methane reforming reaction[J]. Int J Hydrogen Energy, 1997, 22(12):1111-1115. doi: 10.1016/S0360-3199(97)00007-4 [41] YANG M, JIN P, FAN Y, HUANG C, ZHANG N, WENG W, CHEN M, WAN H. Ammonia-assisted synthesis towards a phyllosilicate-derived high-dispersed and long-lived Ni/SiO2 catalyst[J]. Catal Sci Technol, 2015, 5(12):5095-5099. doi: 10.1039/C5CY01361E [42] WO H, DEARN K D, SONG R, HU E, XU Y, HU X. Morphology, composition and structure of carbon deposits from diesel and biomass oil/diesel blends on a pintle-type fuel injector nozzle[J]. Tribol Int, 2015, 91:189-196. doi: 10.1016/j.triboint.2015.07.003 [43] ALEKSANDROV H A, PEGIOS N, PALKOVITS R, SIMENONV K, VAYSSILOV G N. Elucidation of the higher coking resistance of small versus large nickel nanoparticles in methane dry reforming via computational modeling[J]. Catal Sci Technol, 2017, 7(15):3339-3347. doi: 10.1039/C7CY00773F [44] WANG C Z, SUN N N, ZHAO N, WEI W, ZHANG J, ZHAO T J, SUN Y H, SUN C G, LIU H, SNAPE C E. The properties of individual carbon residuals and their influence on the deactivation of Ni-CaO-ZrO2 catalysts in CH4 dry reforming[J]. ChemCatChem, 2014, 6(2):640-648. doi: 10.1002/cctc.v6.2 -





 下载:
下载: