Direct synthesis of ethanol via CO2 hydrogenation over the Co/La-Ga-O composite oxide catalyst
-
摘要: 以LaCo1-xGaxO3为前驱体,还原后得到的Co/La2O3-La4Ga2O9复合氧化物催化剂,用于CO2加氢直接制乙醇。通过XRD、XPS、TPD和TEM等技术对催化剂结构进行了表征,采用微型固定床反应器在230-290℃、3 MPa、空速(GHSV)为3000 mL/(gcat·h)和H2/CO2进料物质的量比为3.0的条件下,考察了该Co/La-Ga-O复合氧化物用于CO2加氢制乙醇的催化性能。结果显示,该Co/La-Ga-O复合氧化物催化剂对生成乙醇具有很高的选择性。与LaCoO3相比,Ga的掺杂可抑制甲烷的形成,促进醇类(特别是乙醇)的生成。当Co/Ga比为7:3时,还原后的LaCo1-xGaxO3催化剂体现出最好的催化性能,CO2转化率为9.8%,总醇选择性达到74.7%,其中,液相产物中的乙醇质量分数可达到88.1%。基于实验结果推测,该催化剂上Co0和Coδ+的协同作用促使CO2选择性加氢生成乙醇。Abstract: A new Co/La2O3-La4Ga2O9 catalyst was prepared by reducing LaCo1-xGaxO3 perovskite and used in the direct synthesis of ethanol from CO2 hydrogenation. The composite catalyst was characterized by XRD, XPS, TPD and TEM and its catalytic performance in CO2 hydrogenation was investigated in a micro fixed-bed reactor operated at 230-290℃, 3 MPa, gas hourly space velocity (GHSV) of 3000 mL/(gcat·h) and H2/CO2 molar ratio of 3.0. The results indicate that the Co/La-Ga-O composite oxide catalyst exhibits high selectivity to ethanol in CO2 hydrogenation. In comparison with the LaCoO3 catalyst, the incorporation of Ga dopant can inhibit the formation of CH4 and then promote the production of alcohols, especially ethanol. With a Co/Ga atomic ratio of 7:3, the Co/La-Ga-O composite oxide catalyst displays the best performance in CO2 hydrogenation, with a CO2 conversion of 9.8%, a selectivity of 74.7% to total alcohols and ethanol content of 88.1% (mass ratio) in the alcohols mixture. On the basis of the experimental results, it is speculated that the synergistic effect of surface Co0 and Coδ+ may contribute to the excellent performance of the Co/La2O3-La4Ga2O9 catalyst in CO2 hydrogenation to ethanol.
-
Key words:
- carbon dioxide hydrogenation /
- ethanol synthesis /
- perovskite-type oxide /
- cobalt /
- gallium
-
图 4 还原后的LaCoO3(■)、LaCo0.8Ga0.2O3(●)、LaCo0.7Ga0.3O3(▲)以及LaCo0.7Ga0.3O3反应100 h后(▶)、LaCo0.6Ga0.4O3(▼)和LaCo0.5Ga0.5O3(◆)样品的N2吸附-脱附等温线(a)和BJH孔径分布曲线(b)
Figure 4 N2 adsorption-desorption isotherms (a) and BJH pore size distribution (b) of the reduced LaCoO3 (■), LaCo0.8Ga0.2O3 (●), LaCo0.7Ga0.3O3 (▲) and LaCo0.7Ga0.3O3 after reaction for 100 h(▶), LaCo0.6Ga0.4O3 (▼) and LaCo0.5Ga0.5O3 (◆) samples
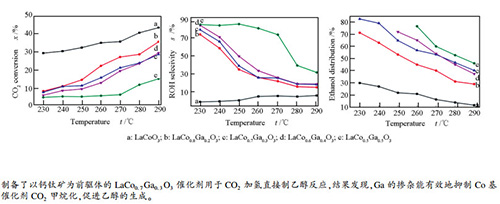
图 9 还原后的LaCoO3(■)、LaCo0.8Ga0.2O3(●)、LaCo0.7Ga0.3O3(▲)、LaCo0.6Ga0.4O3(▼)、LaCo0.5Ga0.5O3(◆)样品的CO2转化率(a)、总醇选择性(b)和乙醇分布(c)随反应温度的变化
Figure 9 CO2 conversion (a), selectivity to alcohols (b), and ethanol distribution (c) as function of reaction temperature on the reduced LaCoO3 (■), LaCo0.8Ga0.2O3 (●), LaCo0.7Ga0.3O3 (▲), LaCo0.6Ga0.4O3 (▼) and LaCo0.5Ga0.5O3 (◆) catalysts
表 1 LaCo1-xGaxO3样品(x=0、0.2、0.3、0.4和0.5)的元素组成、晶粒粒径、耗氢量以及还原度计算
Table 1 Elemental analysis, crystal sizes, hydrogen consumptions and reducibility degree of Con+ in the LaCo1-xGaxO3 samples (x=0, 0.2, 0.3, 0.4 and 0.5)
Sample Co loading/% Co/Gaa Co/Gab Crystal size of Coc, d/nm H2 uptake/
(mmol·g-1)Co dispersion/%c, e H2 consumptions from TPR resultsf, g Total theoretic H2consumptionsg Reducibility degree of Con+/% α β x=0 26.6 - - 18.5 0.104 5.2 0.101 0.204 0.305 100 x=0.2 20.6 4.00 3.97 8.8 0.184 11.5 0.077 0.162 0.242 98.8 x=0.3 17.8 2.33 2.36 7.6(10.5)h 0.188(0.137) 13.4(9.8)h 0.068 0.138 0.211 97.6 x=0.4 15.0 1.50 1.50 6.9 0.173 14.4 0.064 0.122 0.180 103.3 x=0.5 12.3 1.00 1.01 7.4 0.136 13.6 0.051 0.108 0.149 106.7 a: Co/Ga ratio in synthesis system; b: Co/Ga ratio in sample measured from ICP; c: calculated from the results of H2-TPD; d: the crystal size for catalysts after reduction; e: assuming H/Co=1; f: experimental H2 consumptions calculated from TPR results using CuO as the reference material; g: the unit is mmol H2 per 50 mg of catalyst; h: crystal size of Co after 100 h stability test in the parentheses 表 2 还原后LaCo1-xGaxO3样品(x=0、0.2、0.3、0.4和0.5)的物理性质
Table 2 Physical properties of the reduced LaCo1-xGaxO3 samples
Sample Specific surface area A/(m2·g-1) Total pore volume v/(cm3·g-1) Average pore size d/nm x=0 5.6 0.02 4.2 x=0.2 9.6 0.04 27.0 x=0.3 9.4(9.1)a 0.02(0.03)a 25.8(24.4)a x=0.4 8.8 0.02 8.9 x=0.5 6.5 0.02 6.2 a: data in the parentheses are for the spent catalyst after 100 h reaction test 表 3 通过XPS计算所得反应后的LaCo1-xGaxO3样品(x=0和0.3)表面组成
Table 3 Surface atomic ratios calculated by XPS results of the spent LaCo1-xGaxO3 samples (x=0 and 0.3) after reaction
Sample Atomic ratio/% La/Σ[M]a Co/Σ[M]a Ga/Σ[M]a LaCoO3-used 56.1(50.0)b 43.9(50.0)b - LaCo0.7Ga0.3O3-used 47.0(50.0)b 15.5(35.0)b 37.5(15.0)b a: the atomic ratio of metal M is M/(La+Co+Ga) and the data in the parentheses are theoretical values 表 4 LaCo1-xGaxO3(x=0、0.2、0.3、0.4和0.5)系列样品在CO2+H2的催化转化
Table 4 Reaction results of CO2 hydrogenation to alcohols over reduced LaCo1-xGaxO3 catalysts (x=0, 0.2, 0.3, 0.4 and 0.5)a
Sample CO2 conversion x/% Selectivityb sC, mol/% Alcohol distributionc w/% CH4 C2+H ROH MeOH EtOH x=0 30.4 97.8 1.7 0.5 77.0 23.0 x=0.2 10.6 37.4 2.5 60.1 30.2 69.8 x=0.3 9.8 23.1 2.2 74.7 11.9 88.1 x=0.4 8.1 12.2 2.1 85.7 27.5 72.5 x=0.5 4.78 11.1 2.4 86.5 - - a: reaction conditions: 240 ℃, 3 MPa, n(H2)/n(CO2) = 3.0, GSHV = 3000 mL/(gcat·h); the data were obtained after 18 h on stream;
b: product selectivity was based carbon molar quantity, defined as the carbon molar quantity in a carbon-containing product divided by converted carbon moles; C2+H represents hydrocarbons exclusive of methane;
c: alcohol distribution (mass ratio) is the weight fraction of each alcohol in total alcohols -
[1] WANG D, BI Q, YIN G, ZHAO W, HUANG F, XIE X, JIANG M. Direct synthesis of ethanol via CO2 hydrogenation using supported gold catalysts[J]. Chem Commun (Camb), 2016, 52(99): 14226-14229. doi: 10.1039/C6CC08161D [2] WANG W, WANG S, MA X, GONG J. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chem Soc Rev, 2011, 40(7): 3703-3727. doi: 10.1039/c1cs15008a [3] WEI W, JINLONG G. Methanation of carbon dioxide: An overview[J]. Front Chem Sci Eng, 2011, 5(1): 2-10. doi: 10.1007/s11705-010-0528-3 [4] AZIZ M A A, JALIL A A, TRIWAHYONO S, AHMAD A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects[J]. Green Chem, 2015, 17(5): 2647-2663. doi: 10.1039/C5GC00119F [5] POROSOFF M D, YAN B, CHEN J G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities[J]. Energy Environ Sci, 2016, 9(1): 62-73. doi: 10.1039/C5EE02657A [6] SUN K, LU W, QIU F, LIU S, XU X. Direct synthesis of DME over bifunctional catalyst: Surface properties and catalytic performance[J]. Appl Catal A: Gen, 2003, 252(2): 243-249. doi: 10.1016/S0926-860X(03)00466-6 [7] WANG L, WANG L, ZHANG J, LIU X, WANG H, ZHANG W, YANG Q, MA J, DONG X, YOO S J, KIM J, MENG X, XIAO F. Selective hydrogenation of CO2 to ethanol over cobalt catalysts[J]. Angew Chem Int Ed, 2018, 57(21): 6104-6108. doi: 10.1002/anie.201800729 [8] WANG D, BI Q, YIN G, ZHAO W, HUANG F, XIE X, JIANG M. Direct synthesis of ethanol via CO2 hydrogenation using supported gold catalysts[J]. Chem Commun, 2016, 52(99): 14226-14229. doi: 10.1039/C6CC08161D [9] QIAN Q, CUI M, HE Z, WU C, ZHU Q, ZHANG Z, MA J, YANG G, ZHANG J, HAN B. Highly selective hydrogenation of CO2 into C2+ alcohols by homogeneous catalysis[J]. Chem Sci, 2015, 6(10): 5685-5689. doi: 10.1039/C5SC02000J [10] HE Z, QIAN Q, MA J, MENG Q, ZHOU H, SONG J, LIU Z, HAN B. Water-enhanced synthesis of higher alcohols from CO2 Hydrogenation over a Pt/Co3O4 catalyst under milder conditions[J]. Angew Chem Int Ed, 2016, 55(2): 737-741. doi: 10.1002/anie.201507585 [11] OUYANG B, XIONG S, ZHANG Y, LIU B, LI J. The study of morphology effect of Pt/Co3O4 catalysts for higher alcohol synthesis from CO2 hydrogenation[J]. Appl Catal A: Gen, 2017, 543: 189-195. doi: 10.1016/j.apcata.2017.06.031 [12] PRIETO G. Carbon dioxide hydrogenation into higher hydrocarbons and oxygenates: Thermodynamic and kinetic bounds and progress with heterogeneousand homogeneous catalysis[J]. ChemSusChem, 2017, 10(6): 1056-1070. doi: 10.1002/cssc.201601591 [13] GNANAMANI M K, JACOBS G, KEOGH R A, SHAFER W D, SPARKS D E, HOPPS S D, THOMAS G A, DAVIS B H. Fischer-Tropsch synthesis: Effect of pretreatment conditions of cobalt on activity and selectivity for hydrogenation of carbon dioxide[J]. Appl Catal A: Gen, 2015, 499: 39-46. doi: 10.1016/j.apcata.2015.03.046 [14] GNANAMANI M K, HAMDEH H H, JACOBS G, SHAFER W D, HOPPS S D, THOMAS G A, DAVIS B H. Hydrogenation of carbon dioxide over K-promoted FeCo bimetallic catalysts prepared from mixed metal oxalates[J]. ChemCatChem, 2017, 9(7): 1303-1312. doi: 10.1002/cctc.201601337 [15] GUO H, LI S, PENG F, ZHANG H, XIONG L, HUANG C, WANG C, CHEN X. Roles investigation of promoters in K/Cu-Zn catalyst and higher alcohols synthesis from CO2 hydrogenation over a novel two-stage bed catalyst combination system[J]. Catal Lett, 2015, 145(2): 620-630. doi: 10.1007/s10562-014-1446-7 [16] GNANAMANI M K, JACOBS G, HAMDEH H H, SHAFER W D, LIU F, HOPPS S D, THOMAS G A, DAVIS B H. Hydrogenation of carbon dioxide over Co-Fe bimetallic catalysts[J]. ACS Catal, 2016, 6(2): 913-927. doi: 10.1021/acscatal.5b01346 [17] POUR A N, HOSAINI E, IZADYAR M, HOUSAINDOKHT M R. Particle size effects in Fischer-Tropsch synthesis by Co catalyst supported on carbon nanotubes[J]. Chin J Catal, 2015, 36(8): 1372-1378. doi: 10.1016/S1872-2067(15)60840-3 [18] ZHOU G, LIU H, XING Y, XU S, XIE H, XIONG K. CO2 hydrogenation to methane over mesoporous Co/SiO2 catalysts: Effect of structure[J]. J CO2 Util, 2018, 26: 221-229. doi: 10.1016/j.jcou.2018.04.023 [19] LIU H, XU S, ZHOU G, XIONG K, JIAO Z, WANG S. CO2 hydrogenation to methane over Co/KIT-6 catalysts: Effect of Co content[J]. Fuel, 2018, 217: 570-576. doi: 10.1016/j.fuel.2017.12.112 [20] LI Z, SI M, XIN L, LIU R, LIU R, LV J. Cobalt catalysts for Fischer-Tropsch synthesis: The effect of support, precipitant and pH value[J]. Chin J Chem Eng, 2018, 26(4): 747-752. doi: 10.1016/j.cjche.2017.11.001 [21] JOHNSON G R, BELL A T. Effects of Lewis acidity of metal oxide promoters on the activity and selectivity of Co-based Fischer-Tropsch synthesis catalysts[J]. J Catal, 2016, 338: 250-264. doi: 10.1016/j.jcat.2016.03.022 [22] LEE M, JUNG W. Hydrothermal synthesis of LaCO3OH and Ln3+-doped LaCO3OH powders under ambient pressure and their transformation to La2O2CO3 and La2O3[J]. Bull Korean Chem Soc, 2013, 34(12): 3609-3614. doi: 10.5012/bkcs.2013.34.12.3609 [23] CHAKRABARTI D, DE KLERK A, PRASAD V, GNANAMANI M K, SHAFER W D, JACOBS G, SPARKS D E, DAVIS B H. Conversion of CO2 over a Co-based Fischer-Tropsch catalyst[J]. Ind Eng Chem Res, 2015, 54(4): 1189-1196. doi: 10.1021/ie503496m [24] GAO P, LI F, ZHAN H, ZHAO N, XIAO F, WEI W, ZHONG L, WANG H, SUN Y. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. J Catal, 2013, 298: 51-60. doi: 10.1016/j.jcat.2012.10.030 [25] DU H, ZHU H, ZHAO Z, DONG W, LUO W, LU W, JIANG M, LIU T, DING Y. Effects of impregnation strategy on structure and performance of bimetallic CoFe/AC catalysts for higher alcohols synthesis from syngas[J]. Appl Catal A: Gen, 2016, 523: 263-271. doi: 10.1016/j.apcata.2016.06.022 [26] BEDEL L, ROGER A C, ESTOURNES C, KIENNEMANN A. Co0 from partial reduction of La(Co, Fe)O3 perovskites for Fischer-Tropsch synthesis[J]. Catal Today, 2003, 85(2/4): 207-218. http://www.sciencedirect.com/science/article/pii/S0920586103003882 [27] TONIOLO F S, MAGALHÃES R N S H, PEREZ C A C, SCHMAL M. Structural investigation of LaCoO3 and LaCoCuO3 perovskite-type oxides and the effect of Cu on coke deposition in the partial oxidation of methane[J]. Appl Catal B: Environ, 2012, 117/118: 156-166. doi: 10.1016/j.apcatb.2012.01.009 [28] ONRUBIA J A, PEREDA-AYO B, DE-LA-TORRE U, GONZÁLEZ-VELASCO J R. Key factors in Sr-doped LaBO3 (B=Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification[J]. Appl Catal B: Environ, 2017, 213: 198-210. doi: 10.1016/j.apcatb.2017.04.068 [29] NIU T, LIU G L, CHEN Y, YANG J, WU J, CAO Y, LIU Y. Hydrothermal synthesis of graphene-LaFeO3 composite supported with Cu-Co nanocatalyst for higher alcohol synthesis from syngas[J]. Appl Surf Sci, 2016, 364: 388-399. doi: 10.1016/j.apsusc.2015.12.164 [30] GUO S, LI S, ZHONG H, GONG D, WANG J, KANG N, ZHANG L, LIU G, LIU Y. Mixed oxides confined and tailored cobalt nanocatalyst for direct ethanol synthesis from syngas: A catalyst designing by using Perovskite-Type oxide as the precursor[J]. Ind Eng Chem Res, 2018, 57(6): 2404-2415. doi: 10.1021/acs.iecr.7b04336 [31] PODILA S, DRISS H, ZAMAN S F, ALHAMED Y A, ALZAHRANI A A, DAOUS M A, PETROV L A. Hydrogen generation by ammonia decomposition using Co/MgO-La2O3 catalyst: Influence of support calcination atmosphere[J]. J Mol Catal A: Chem, 2016, 414: 130-139. doi: 10.1016/j.molcata.2016.01.012 [32] DE LA PEÑA O SHEA V A, GONZÁLEZ S, ILLAS F, FIERRO J L G. Evidence for spontaneous CO2 activation on cobalt surfaces[J]. Chem Phys Lett, 2008, 454(4/6): 262-268. http://www.sciencedirect.com/science/article/pii/S0009261408001759 [33] YAZDANI P, WANG B, GAO F, KAWI S, BORGNA A. Role of the strong lewis base sites on glucose hydrogenolysis[J]. ChemCatChem, 2018, 10(17): 3845-3853. doi: 10.1002/cctc.201800427 [34] PODILA S, DRISS H, ZAMAN S F, ALI A M, AL-ZAHRANI A A, DAOUS M A, PETROV L A. Effect of preparation methods on the catalyst performance of Co/Mg La mixed oxide catalyst for COx-free hydrogen production by ammonia decomposition[J]. Int J Hydrogen Energy, 2017, 42(38): 24213-24221. doi: 10.1016/j.ijhydene.2017.07.112 [35] ZHANG Y, HAN K, CHENG T, FANG Z. Synthesis, characterization, and photoluminescence property of LaCO3OH microspheres[J]. Inorg Chem, 2007, 46(11): 4713-4717. doi: 10.1021/ic0701458 [36] JENA H, GOVINDAN KUTTY K V, KUTTY T R N. Novel wet chemical synthesis and ionic transport properties of LaGaO3 and selected doped compositions at elevated temperatures[J]. Mater Sci Eng B, 2004, 113(1): 30-41. doi: 10.1016/j.mseb.2004.06.017 [37] JAIN R, GOPINATH C S. New strategy toward a dual functional nanocatalyst at ambient conditions: Influence of the Pd-Co interface in the catalytic activity of Pd@Co core-shell nanoparticles[J]. ACS Appl Mater Interfaces, 2018, 10(48): 41268-41278. doi: 10.1021/acsami.8b12940 [38] BIESINGER M C, PAYNE B P, GROSVENOR A P, LAU L W M, GERSON A R, SMART R S C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni[J]. Appl Surf Sci, 2011, 257(7): 2717-2730. doi: 10.1016/j.apsusc.2010.10.051 [39] LIU L F, KANG J F, WANG Y, TANG H, KONG L G, SUN L, ZHANG X, HAN R Q. The influence of hydrogen annealing on magnetism of Co-doped TiO2 films prepared by sol-gel method[J]. J Magn Magn Mater, 2007, 308(1): 85-89. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6aa40746153718f5fd0c5864a7ac3240 [40] ZHAO L, HAN T, WANG H, ZHANG L, LIU Y. Ni-Co alloy catalyst from LaNi1-xCoxO3 perovskite supported on zirconia for steam reforming of ethanol[J]. Appl Catal B: Environ, 2016, 187: 19-29. doi: 10.1016/j.apcatb.2016.01.007 [41] SAN-JOSÉ-ALONSO D, JUAN-JUAN J, ILLÁN-GÍMEZ M J, ROMÁN-MARTÍNEZ M C. Ni, Co and bimetallic Ni-Co catalysts for the dry reforming of methane[J]. Appl Catal A: Gen, 2009, 371(1/2): 54-59. http://www.sciencedirect.com/science/article/pii/S0926860X09006486 [42] LIU F, ZHAO L, WANG H, BAI X, LIU Y. Study on the preparation of Ni-La-Ce oxide catalyst for steam reforming of ethanol[J]. Int J Hydrogen Energy, 2014, 39(20): 10454-10466. doi: 10.1016/j.ijhydene.2014.05.036 [43] YANG Q, CAO A, KANG N, AN K, LIU Z, LIU Y. A new catalyst of Co/La2O3-doped La4Ga2O9 for direct ethanol synthesis from syngas[J]. Fuel Process Technol, 2018, 179: 42-52. doi: 10.1016/j.fuproc.2018.06.011 [44] PEI Y, LIU J, ZHAO Y, DING Y, LIU T, DONG W, ZHU H, SU H, YAN L, LI J, LI W. High alcohols synthesis via Fischer-Tropsch reaction at cobalt metal/carbide interface[J]. ACS Catal, 2015, 5(6): 3620-3624. doi: 10.1021/acscatal.5b00791 [45] R W DORNER D R H F, WILLAUER H D. Influence of gas feed composition and pressure on the catalytic conversion of CO2 to hydrocarbons using a traditional cobalt-based Fischer-Tropsch catalyst[J]. Energy Fuels, 2009, 23: 4190-4195. doi: 10.1021/ef900275m [46] JIAO G, DING Y, ZHU H, LI X, LI J, LIN R, DONG W, GONG L, PEI Y, LU Y. Effect of La2O3 doping on syntheses of C1-C18 mixed linear α-alcohols from syngas over the Co/AC catalysts[J]. Appl Catal A: Gen, 2009, 364(1/2): 137-142. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=646f4f80b9f060d1ffc633530adcb340 [47] SMITH M L, KUMAR N, SPIVEY J J. CO adsorption behavior of Cu/SiO2, Co/SiO2, and CuCo/SiO2 catalysts studied by in situ DRIFTS[J]. J Phys Chem C, 2012, 116(14): 7931-7939. doi: 10.1021/jp301197s [48] ZHANG Y, JACOBS G, SPARKS D E, DRY M E, DAVIS B H. CO and CO2 hydrogenation study on supported cobalt Fischer-Tropsch synthesis catalysts[J]. Catal Today, 2002, 71(3/4): 411-418. http://www.sciencedirect.com/science/article/pii/S0920586101004680 [49] TAKANABE K, NAGAOKA K, NARIAI K, AIKA K. Titania-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane[J]. J Catal, 2005, 232(2): 268-275. doi: 10.1016/j.jcat.2005.03.011 -





 下载:
下载: