Effect of promoter M(M=Cr, Zn, Y, La) on CuO/CeO2 catalysts for hydrogen production from steam reforming of methanol
-
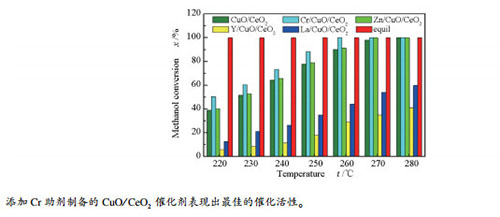
摘要: 采用顺序浸渍法制备了掺杂助剂M(M=Cr、Zn、Y、La)的CuO/CeO2催化剂,并利用XRF、XRD、BET、H2-TPR和XPS等手段对催化剂进行了表征,考察了不同助剂对CuO/CeO2催化剂结构、性质和性能的影响。结果表明,助剂的掺杂主要影响CuO/CeO2催化剂的CuO分散、催化剂的还原性质、CuO与CeO2间的相互作用和催化剂表面氧空穴含量。掺杂助剂Cr和Zn后,提高了CuO在催化剂表面的分散度,使CuO和CeO2间的相互作用加强,表面氧空穴增加,进而使得催化活性提高。而掺杂助剂Y和La后,降低了CuO在催化剂表面的分散度,使CuO和CeO2间的相互作用减弱,表面氧空穴减少,进而使得催化活性降低。其中,掺杂Cr助剂的催化剂催化性能较优,当反应条件为260 ℃,n(CH3OH):n(H2O)=1:1.2,甲醇水蒸气气体空速为1760 h-1时,最终转化率可达100%,重整尾气中CO含量为0.15%,与CuO/CeO2催化剂相比,转化率提高了10%,重整尾气中CO含量降低了0.34%。Abstract: M/CuO/CeO2 (M=Cr, Zn, Y, La) catalyst was prepared by sequential impregnation method. The catalysts were characterized by XRF, XRD, BET, H2-TPR and XPS. The effects of different promoters on the structure and properties of CuO/CeO2 catalysts were investigated.The results show that the doping of promoters mainly affects the dispersion of CuO, the reduction properties of the catalyst, the interaction between CuO and CeO2, and the oxygen hole content on the surface of the catalyst. After doping additives Cr and Zn, improving the dispersion of CuO on catalysts, and the interaction between CuO and CeO2 strengthens, the surface oxygen holes increase, which in turn increases the catalytic activity. After doping the additives Y and La, decreasing the dispersion of CuO on catalysts, the interaction between CuO and CeO2 is weakened, and the surface oxygen holes are reduced, thus the catalytic activity is reduced. Among them, the catalyst doped with promoter Cr has better catalytic activity. When the reaction conditions are 260℃, n(CH3OH):n(H2O)=1:1.2 and the space velocity of methanol vapor gas is 1760 h-1, the final conversion can reach 100%, the CO content in reforming tail gas is 0.15%. Compared with CuO/CeO2 catalyst, the conversion rate is increased by 10%, and the CO content in reforming tail gas is reduced by 0.34%.
-
Key words:
- auxiliaries /
- methanol steam reforming /
- hydrogen /
- carbon monoxide
-
表 1 不同助剂催化剂的元素含量
Table 1 Element content of catalysts with different auxiliaries
Catalyst Content of element w/% Cu Ce O Cr Zn Y La CuO/CeO2 7.9 73.4 18.7 - - - - Cr/CuO/CeO2 7.3 70.7 19.3 2.7 - - - Zn/CuO/CeO2 7.2 71.4 18.8 - 2.6 - - Y/CuO/CeO2 7.1 71.5 18.8 - - 2.6 - La/CuO/CeO2 7.3 71.4 18.6 - - - 2.7 表 2 催化材料的物化性质和产氢速率
Table 2 Physicochemical properties and hydrogen production rate of catalytic materials
Catalyst Specific surface area A/(m2·g-1) Pore volume v/(cm3·g-1) dCuO/nm Cu dispersiona/% Cu surface areaa A/(m2·g-1) H2 production rateb/(cm3·kg-1·s-1) CeO2 37.4 0.10 - - - - CuO/CeO2 21.9 0.09 29.9 15.3 8.8 379.7 Cr/CuO/CeO2 18.6 0.08 20.2 16.8 9.7 631.2 Zn/CuO/CeO2 28.2 0.11 22.5 16.5 9.5 521.8 Y/CuO/CeO2 18.6 0.08 25.4 14.1 8.1 282.5 La/CuO/CeO2 27.5 0.07 23.8 15.1 8.7 334.7 a: determined by N2O experiments; b: reaction conditions: 280 ℃, n(CH3OH):n(H2O)=1.2:1, GHSV=1760 h-1 表 3 催化剂的还原峰位置
Table 3 Reduction peak position of catalyst
Catalyst Peak position t/ ℃ peak α peak β peak γ CuO/CeO2 178 222 249 Cr/CuO/CeO2 150 205 235 Zn/CuO/CeO2 160 204 217 Y/CuO/CeO2 247 264 285 La/CuO/CeO2 203 252 272 表 4 催化剂的Ce 3d和Cu LMM XPS曲线拟合结果
Table 4 Fitting results of Cu LMM and Ce 3d XPS curves of catalysts
Catalyst Ce3+/(Ce3++Ce4+)/% Cu+/(Cu++Cu2+)/% CuO/CeO2 18.5 43.9 Cr/CuO/CeO2 34.4 74.9 Zn/CuO/CeO2 31.5 46.4 Y/CuO/CeO2 13.5 31.5 La/CuO/CeO2 15.9 40.2 -
[1] HOU H J M. Hydrogen energy production using manganese/semiconductor system inspired by photosynthesisInt[J]. J Hydrogen Energy, 2017, 42(12): 8530-8538. doi: 10.1016/j.ijhydene.2017.01.100 [2] WEI Z, KE Y, PEI P, YU W, CHU X Y, LI S L, YANG K. Hydrogen production from cylindrical methanol steam reforming microreactor with porous Cu-Al fiber sintered felt[J]. Int J Hydrogen Energy, 2018, 43(7): 3643-3654. doi: 10.1016/j.ijhydene.2017.12.118 [3] XI H, HOU X, LIU Y, QING S J, GAO Z X. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angew Chem, 2015, 53(44): 11886-11889. [4] HAO C, ANDOLINA C M, LI J, CURNAN M T, SAIDI W A, ZHOU G W, YANG J C, VESER G. Dependence of H2 and CO2 selectivity on Cu oxidation state during partial oxidation of methanol on Cu/ZnO[J]. Appl Catal A: Gen, 2018, 556: 64-72. doi: 10.1016/j.apcata.2018.02.028 [5] JAMPA S, JAMIESON A M, CHAISUWAN T, LUENGNARUEMITCHAI A, WONGKASEMJIT S. Achievement of hydrogen production from autothermal steam reforming of methanol over Cu-loaded mesoporous CeO2 and Cu-loaded mesoporous CeO2-ZrO2 catalysts[J]. Int J Hydrogen Energy, 2017, 42(22): 15073-15084. doi: 10.1016/j.ijhydene.2017.05.022 [6] LIU Y, HAYAKAWA T, TSUNODA T, SUZUKI K, HAMAKAWA S, MURATA K, SHIOZAKI R, ISHⅡ T, KUMAGAI M. Steam reforming of methanol over Cu/CeO2 catalysts studied in comparison with Cu/ZnO and Cu/Zn(Al)O catalysts[J]. Top Catal, 2003, 22(3/4): 205-213. doi: 10.1023/A:1023519802373 [7] LI Y F, DONG X F, LIN W M. Effects of ZrO2-promoter on catalytic performance of CuZnAlO catalysts for production of hydrogen by steam reforming of methanol[J]. Int J Hydrogen Energy, 2004, 29(15): 1617-1621. doi: 10.1016/j.ijhydene.2004.03.001 [8] PAPAVASILIOU J, AVGOUROPOULOS G, IOANNIDES T. Effect of dopants on the performance of CuO-CeO2 catalysts in methanol steam reforming[J]. Appl Catal B: Environ, 2007, 69(3/4): 226-234. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5b9a1b2456f04891db3033b15ff1dd11 [9] ZAHEDI T O, MAJID T, DEHGHANI K A. Methanol steam reforming in a microchannel reactor by Zn-, Ce- and Zr- modified mesoporous Cu/SBA-15 nanocatalyst[J]. Int J Hydrogen Energy, 2018, 43(31): 14103-14120. doi: 10.1016/j.ijhydene.2018.06.035 [10] YANG S Q, ZHOU F, LIU Y J, ZHANG L, YU C, WANG H H, TIAN Y, ZHANG C S, LIU D S. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy, 2019, 44(14): 7252-7261. doi: 10.1016/j.ijhydene.2019.01.254 [11] 刘玉娟, 王东哲, 张磊, 王宏浩, 陈琳, 刘道胜, 韩蛟, 张财顺.载体焙烧气氛对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J].燃料化学学报, 2018, 46(8): 992-999. doi: 10.3969/j.issn.0253-2409.2018.08.011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, WANG Hong-hao, CHEN Lin, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Effect of support calcination atmospheres on the activity of CuO/CeO2 catalysts for methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(8): 992-999. doi: 10.3969/j.issn.0253-2409.2018.08.011 [12] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭.稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(2): 179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lei, YANG Zhan-xu. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(2): 179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007 [13] 杨淑倩, 张娜, 贺建平, 张磊, 王宏浩, 白金, 张健, 刘道胜, 杨占旭. Ce的浸渍顺序对Cu/Zn-Al水滑石衍生催化剂用于甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(4): 479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014YANG Shu-qian, ZHANG Na, HE Jian-ping, ZHANG Lei, WANG Hong-hao, BAI Jin, ZHANG Jian, LIU Dao-sheng, YANG Zhan-xu. Effect of impregnation sequence of Ce on the performance of Cu/ Zn-Al catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(4): 479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014 [14] HE J P, YANG Z X, ZHANG L, LI Y, PAN L W. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming[J]. Int J Hydrogen Energy, 2017, 42(15): 9930-9937. doi: 10.1016/j.ijhydene.2017.01.229 [15] DAI B, ZHOU G, GE S, XIE H M, JIAO Z J, ZHANG G Z, XIONG K. CO2 reverse water-gas shift reaction on mesoporous M-CeO2 catalysts[J]. Can J Chem Eng, 2017, 95(4): 634-642. doi: 10.1002/cjce.22730 [16] 贺建平, 张磊, 陈琳, 杨占旭, 佟宇飞. CeO2改性Cu/Zn-Al水滑石衍生催化剂对甲醇水蒸气重整制氢性能的影响[J].高等学校化学学报, 2017, 38: 1822-1828. doi: 10.7503/cjcu20170158HE Jian-ping, ZHANG Lei, CHEN Lin, YANG Zhan-xu, TONG Yu-fei. Effect of CeO2 on Cu/Zn-Al catalysts derived from hydrotalcite precursor for methanol steam reforming[J]. Chem J Chin Univ, 2017, 38: 1822-1828. doi: 10.7503/cjcu20170158 [17] ZHANG L, PAN L W, NI C J, SUN T J, ZHAO S S, WANG S D, WANG A J, HU Y K. CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming[J]. Int J Hydrogen Energy, 2013, 38(11): 4397-4406. doi: 10.1016/j.ijhydene.2013.01.053 [18] SHE W, QI T, CUI M, YAN P, NG S. W, LI W, LI G. Catalytic performance of CeO2-supported Ni catalyst for hydrogenation of nitroarenes fabricated via coordination-assisted strategy[J]. ACS Appl Mater Inter, 2018, 10(17): 14698-14707. doi: 10.1021/acsami.8b01187 [19] ZHANG Y, ZHOU Y, PENG C, SHI J, WANG Q, HE L, SHI L. Enhanced activity and stability of copper oxide/γ-alumina catalyst in catalytic wet-air oxidation: Critical roles of cerium incorporation[J]. Appl Surf Sci, 2018, 436: 981-988. doi: 10.1016/j.apsusc.2017.12.036 [20] PENG X, OMASTA T J, ROLLER J M, MUSTAIN W E. Highly active and durable Pd-Cu catalysts for oxygen reduction in alkaline exchange membrane fuel cells[J]. Frontiers Energy, 2017, 11(3): 299-309. doi: 10.1007/s11708-017-0495-1 [21] BENNICI S, GERVASINI A, RAVASIO N, ZACCHERIA F. Optimization of tailoring of CuOx species of silica alumina supported catalysts for the selective catalytic reduction of NOx[J]. J Phys Chem B, 2003, 107(22): 5168-5176. doi: 10.1021/jp022064x [22] FANG Z, REHMAN S U, SUN M, YUAN Y P, JIN S W, HONG B. Hybrid NiO-CuO mesoporous nanowire array with abundant oxygen vacancies and a hollow structure as a high-performance asymmetric supercapacitor[J]. J Mater Chem A, 2018, 6: 21131-21142. doi: 10.1039/C8TA08262F [23] AFONASENKO T N, TSYRULNIKOV P G, GULYAEVA T I, LEONTEVA N N, SMIRNOVA N S, KOCHUBEI D I, SUPRUN E A, SALANOV A N. (CuO-CeO2)/glass cloth catalysts for selective CO oxidation in the presence of H2: The effect of the nature of the fuel component used in their surface self-propagating high-temperature synthesis on their properties[J]. Kinet Catal, 2013, 54(1): 59-68. doi: 10.1134/S0023158412060018 [24] KULKARNI G U, RAO C N R. EXAFS and XPS investigations of Cu/ZnO catalysts and their interaction with CO and methanol[J]. Top Catal, 2003, 22(3): 183-189. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c18db8e5a8333691cda5a303df6a1fb8 [25] ESPINOS J P, MORALES J, BARRANCO A, CABALLERO A, HOLGADO J P, GONZALES-ELIPE A R. Interface effects for Cu, CuO, and Cu2O deposited on SiO2 and ZrO2. XPS determination of the valence state of copper in Cu/SiO2 and Cu/ZrO2 catalysts[J]. J Phys Chem B, 2002, 106(27): 6921-6929. doi: 10.1021/jp014618m [26] NATILE M M, GALENDA A, GLISENTI A. CuO/CeO2 nanocomposites: An XPS study[J]. Surf Sci Spectra, 2009, 16(1): 13-26. doi: 10.1116/11.20061005 -





 下载:
下载: