Effect of Ce/Zr molar ratio on the catalytic performance of Ru/CexZr1-xO2 in the wet air oxidation of phenol
-
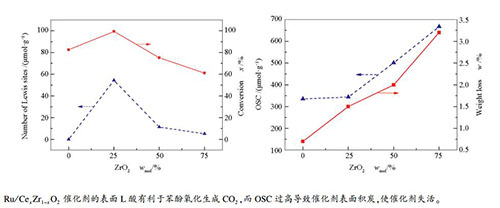
摘要: 采用溶胶凝胶法制备了系列不同Ce/Zr物质的量比的Ru/CexZr1-xO2催化剂,通过X射线衍射(XRD)、氮气吸附-脱附、拉曼(Raman)光谱、储氧能力(oxygen storage capacity,OSC)、热重(TG)以及吡啶红外(Py-FTIR)等手段对其进行了表征,考察了该催化剂在湿式氧化苯酚反应中的性能。结果表明,ZrO2可与CeO2形成固溶体;随着ZrO2掺杂量的增加,CexZr1-xO2固溶体的OSC值增大。相比于CeO2,掺杂ZrO2后催化剂表面的L酸量明显增多。催化剂湿式氧化(catalytic wet air oxidation,CWAO)性能与OSC和表面酸性均有密切的关系:催化剂表面的L酸有利于苯酚氧化生成CO2,而OSC过高会导致催化剂表面积炭,使催化剂失活。当ZrO2掺杂量为25%时,在160℃、2 MPa纯氧条件下,催化氧化苯酚5 h后,苯酚转化率和总有机碳(total organic carbon,TOC)去除率分别为100%和99%,说明该催化剂具有优异的苯酚氧化性能。Abstract: A series of CexZr1-xO2 catalysts with different Ce/Zr molar ratios were prepared by sol-gel method and characterized by X-ray diffraction (XRD), nitrogen sorption, Raman spectra, oxygen storage capacity (OSC), thermogravimetry (TG) and pyridine adsorption infrared (Py-FTIR) spectra; the performance of CexZr1-xO2 in catalytic wet air oxidation (CWAO) of phenol was investigated. The results demonstrate that CexZr1-xO2 solid solution is formed by doping ZrO2 in CeO2 and the OSC value of CexZr1-xO2 increases with an increase in the content of ZrO2. In comparison with pure CeO2, CexZr1-xO2 solid solution has more Lewis acid sites. The catalytic activity of CexZr1-xO2 is related to both OSC and surface acidity; the Lewis acid sites are favorable for the complete oxidation of phenol, whereas high OSC may promote the carbonaceous deposition that leads to catalyst deactivation. The Ru/Ce0.75Zr0.25O2 catalyst exhibits high activity in phenol oxidation; after reaction for 5 h at 160℃ and 2 MPa O2, phenol conversion and total organic carbon (TOC) rate reach 100% and 99%, respectively.
-
Key words:
- catalytic wet air oxidation /
- Ce-Zr solid solution /
- phenol /
- oxygen storage capacity /
- acidity /
- carbon deposit
-
表 1 不同铈锆物质的量比Ru/CexZr1-xO2的晶体大小和比表面积
Table 1 Crystallize size and surface area of Ru/CexZr1-xO2 catalysts with different Ce/Zr molar
Sample Crystalline phase Particle size d/nm Lattice constant d/nm BET surface area A/(m2·g-1) Ru/ZrO2 t+m 16.2 0.518 9.7 Ru/Ce0.25Zr0.75O2 t 8.7 0.520 18.4 Ru/Ce0.5Zr0.5O2 c 6.0 0.534 47.5 Ru/Ce0.75Zr0.25O2 c 8.4 0.535 71.5 Ru/CeO2 c 33.1 0.541 16.1 notes:′m′ represents for monoclinic crystalline phase, ′t′ represents for tetragonal crystalline phase 表 2 不同铈锆物质的量比Ru/CexZr1-xO2的Raman数据
Table 2 Raman spectra results of various Ru/CexZr1-xO2 catalysts with different Ce/Zr molar ratios
Sample IF2g ID ID/IF2g Ru/ZrO2 - - - Ru/Ce0.25Zr0.75O2 - - - Ru/Ce0.5Zr0.5O2 82395 37608 0.46 Ru/Ce0.75Zr0.25O2 61104 10081 0.16 Ru/CeO2 749845 82483 0.11 表 3 不同铈锆物质的量比Ru/CexZr1-xO2的OSC、L酸量和失重百分比
Table 3 OSC value, amount of Lewis acid sites and weight loss of various Ru/CexZr1-xO2 catalysts
Sample OSC/(μmol·g-1) Number of Lewis acid sites/(μmol·g-1) Weight loss/% Ru/ZrO2 248 81.37 2.6 Ru/Ce0.25Zr0.75O2 667 5.19 3.2 Ru/Ce0.5Zr0.5O2 501 11.36 2.0 Ru/Ce0.75Zr0.25O2 344 54.35 1.5 Ru/CeO2 335 0.00 0.7 -
[1] LEVEC J, PINTAR A. Catalytic wet-air oxidation processes:A review[J]. Catal Today, 2007, 124(3/4):172-184. http://linkinghub.elsevier.com/retrieve/pii/S092058610700199X [2] IMAMURA S. Catalytic and noncatalytic wet oxidation[J]. Ind Eng Chem Res, 1999, 38(5):1743-1753. doi: 10.1021/ie980576l [3] BUSCA G, BERARDINELLI S, RESINI C, ARRIGHI L. Technologies for the removal of phenol from fluid streams:A short review of recent developments[J]. J Hazard Mater, 2008, 160(2/3):265-288. doi: 10.1007%2Fs13201-014-0176-8 [4] CYBULSKI A. Catalytic wet air oxidation:Are monolithic catalysts and reactors feasible?[J]. Ind Eng Chem Res, 2007, 46(12):4007-4033. doi: 10.1021/ie060906z [5] BHARGAVA S K, TARDIO J, PRASAD J, FOGER K, AKOLEKAR D B, GROCOTT S C. Wet oxidation and catalytic wet oxidation[J]. Ind Eng Chem Res, 2006, 45(4):1221-1258. doi: 10.1021/ie051059n [6] BARBIER-JR J, DELANOE F, JABOUILLE F, DUPREZ D, BLANCHARD G, ISNARD P. Total oxidation of acetic acid in aqueous solutions over noble metal catalysts[J]. J Catal, 1998, 177(2):378-385. doi: 10.1006/jcat.1998.2113 [7] OLIVIERO L, BARBIER-JR J, LABRUQUERE S, DUPREZ D. Role of the metal-support interface in the total oxidation of carboxylic acids over Ru/CeO2 catalysts[J]. Catal Lett, 1999, 60(1/2):15-19. doi: 10.1023/A:1019026100843 [8] OLIVIERO L, BARBIER-JR J, DUPREZ D, GUERRERO-RUIZ A, BACHILLER-BAEZA B, RODRIGUEZ-RAMOS I. Catalytic wet air oxidation of phenol and acrylic acid over Ru/C and Ru-CeO2/C catalysts[J]. Appl Catal B:Environ, 2000, 25(4):267-275. doi: 10.1016/S0926-3373(99)00141-1 [9] TROVARELLI A. Catalytic properties of ceria and CeO2-containing materials[J]. Catal Rev, 1996, 38(4):439-520. doi: 10.1080/01614949608006464 [10] KASPAR J, FORNASIERO P, GRAZIANI M. Use of CeO2-based oxides in the three-way catalysis[J]. Catal Today, 1999, 50(2):285-298. doi: 10.1016/S0920-5861(98)00510-0 [11] MONTE R D, KAŠPAR J. Nanostructured CeO2-ZrO2 mixed oxides[J]. J Mater Chem, 2005, 15(6):633-648. doi: 10.1039/B414244F [12] YUAN F G. Synthesis of cerium-zirconium solid solution and their catalytic performance for carbon monoxide oxidation[D]. Yunnan: Yunnan University, 2015. [13] LI N, DESCORME C, BESSON M. Catalytic wet air oxidation of 2-chlorophenol over Ru loaded CexZr1-xO2 solid solutions[J]. Appl Catal B:Environ, 2007, 76(1/2):92-100. https://www.researchgate.net/publication/6319554_Catalytic_Wet_Air_Oxidation_of_2-Chlorophenol_over_Supported_Ruthenium_Catalysts [14] KEAV S, DE LOS MONTEROS A E, BARBIER J, DUPREZ D. Wet air oxidation of phenol over Pt and Ru catalysts supported on cerium-based oxides:Resistance to fouling and kinetic modelling[J]. Appl Catal B:Environ, 2014, 150/151:402-410. doi: 10.1016/j.apcatb.2013.12.028 [15] LAFAYE G, BARBIER J, DUPREZ D. Impact of cerium-based support oxides in catalytic wet air oxidation:Conflicting role of redox and acid-base properties[J]. Catal Today, 2015, 253:89-98. doi: 10.1016/j.cattod.2015.01.037 [16] NOUSIR S, KEAV S, BARBIER J, BENSITEL M, BRAHMI R, DUPREZ D. Deactivation phenomena during catalytic wet air oxidation (CWAO) of phenol over platinum catalysts supported on ceria and ceria-zirconia mixed oxides[J]. Appl Catal B:Environ, 2008, 84(3/4):723-731. https://www.researchgate.net/publication/223514820_Deactivation_phenomena_during_catalytic_wet_air_oxidation_CWAO_of_phenol_over_platinum_catalysts_supported_on_ceria_and_ceria-zirconia_mixed_oxides [17] GANDHE A R, FERNANDES J B, VARMA S, GUPTA N M. TiO2:As a versatile catalyst for the ortho-selective methylation of phenol[J]. J Mol Catal A:Chem, 2005, 238(1/2):63-71. http://linkinghub.elsevier.com/retrieve/pii/S1381116905003298 [18] CHEN Y, DOU H, YANG M, GAO X, WU M, CUI G, SUN C. Study of catalytic wet air oxidation to be used in the pretreatmrnt of phenol wastewater[J]. Ind Water Treat, 2002, 22(6):19-22. doi: 10.1007%2Fs40726-016-0035-3 [19] ROSSIGNOL S, GERARD F, DUPREZ D. Effect of the preparation method on the properties of zirconia-ceria materials[J]. J Mater Chem, 1999, 9(7):1615-1620. doi: 10.1039/a900536f [20] WANG L, WANG S, ZHANG L. Effect of Ce-Zr ratio on properties of ceria-zirconia solid solution as oxygen storage material[J]. Chin J Rare Metals, 2011, 35(2):276-280. [21] HOMSI D, AOUAD S, EL NAKAT J, EL KHOURY B, OBEID P, ABI-AAD E, ABOUKAÏS A. Carbon black and propylene oxidation over Ru/CexZr1-xO2 catalysts[J]. Catal Commun, 2011, 12(8):776-780. doi: 10.1016/j.catcom.2011.01.014 [22] ROSSIGNOL S, MADIER Y, DUPREZ D. Preparation of zirconia-ceria materials by soft chemistry[J]. Catal Today, 1999, 50(2):261-270. doi: 10.1016/S0920-5861(98)00508-2 [23] WU H. Combustion of toluene catalyzed by ceria-zirconia solid solutions[D]. Shandong: China University of Petroleum, 2015. [24] HUANG H, DAI Q, WANG X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene[J]. Appl Catal B:Environ, 2014, 158/159:96-105. doi: 10.1016/j.apcatb.2014.01.062 [25] MAR S Y, CHEN C S, HUANG Y S, TIONG K K. Characterization of RuO2 thin films by Raman spectroscopy[J]. Appl Surf Sci, 1995, 90(4):497-504. doi: 10.1016/0169-4332(95)00177-8 [26] WANG F. Modulation of support microstructure for Ru-based catalysts and their catalytic performance toward CO2 methanation[D]. Beijing: Beijing University of Chemical Technology, 2016. [27] ZHAN Y, CAI G, XIAO Y, ZHENG Q, WEI K. Correlation of storage capacity and properties for Ce-Zr solid solution[J]. Spectrosc Spect Anal, 2007, 12(11):2266-2269. [28] MONTEROS A E D L, LAFAYE G, CERVANTES A, DEL ANGEL G, BARBIER JR J, TORRES G. Catalytic wet air oxidation of phenol over metal catalyst (Ru, Pt) supported on TiO2-CeO2 oxides[J]. Catal Today, 2015, 258:564-569. doi: 10.1016/j.cattod.2015.01.009 [29] CUTRUFELLO M G, FERINO I, MONACI R, ROMBI E, SOLINAS V. Acid-base properties of zirconium, cerium and lanthanum oxides by calorimetric and catalytic investigation[J]. Top Catal, 2002, 19(3/4):225-239. doi: 10.1023/A:1015376409863 [30] ALNAIZY R, AKGERMAN A. Advanced oxidation of phenolic compounds[J]. Adv Environ Res, 2000, 4:233-244. doi: 10.1016/S1093-0191(00)00024-1 [31] MASENDE Z P G, KUSTER B F M, PTASINSKI K J, JANSSEN F J J G, KATIMA J H Y, SCHOUTEN J C. Platinum catalysed wet oxidation of phenol in a stirred slurry reactor:The role of oxygen and phenol loads on reaction pathways[J]. Catal Today, 2003, 79/80:357-370. doi: 10.1016/S0920-5861(03)00064-6 -





 下载:
下载: