Composition and structural characteristics of nitrogen-containing species in the soluble organic species of Xianfeng lignite
-
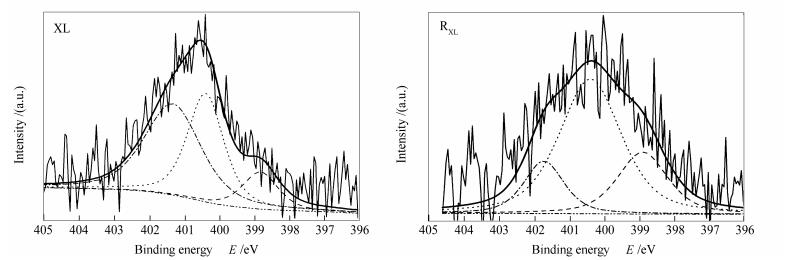
摘要: 先锋褐煤在高压釜中用等体积的甲醇/甲苯溶剂300℃下热溶得到热溶物和热溶残渣,利用X射线光电子能谱(XPS)分析了先锋褐煤及其热溶残渣中氮的形态,利用气相色谱/质谱(GC/MS)和电喷雾傅里叶变换-离子回旋共振质谱(ESI FT-ICR MS)分析了热溶物中含氮化合物的组成和结构特征。研究表明,先锋褐煤中氮形态含量顺序为季氮>吡啶氮>吡咯氮,而季氮在热溶过程中更易溶出。GC/MS共检测出热溶物中20种含氮化合物,并且大部分为胺类化合物。ESI FT-ICR MS检测出热溶物中300多种含氮化合物,大部分含氮化合物含一个或三个氮原子。含一个氮原子的含氮化合物主要以N1O1、N1O2和N1OxS1-2类化合物为主,而含三个氮原子的含氮化合物主要以N3OxS1-2(x=1-12)类化合物为主。含一个氮原子的含氮化合物的等效双键数和碳数随氧原子数增加而增加。Abstract: Xianfeng lignite (XL) was firstly thermal dissolved in the isometric toluene/methanol mixed solvent at 300℃ in a stainless-steel autoclave to afford a soluble portion (SPXL) and a residue (RXL), then the nitrogen forms in XL and RXL were characterized with X-ray photoelectron spectroscopy (XPS) analysis, and the nitrogen-containing species (NCSs) in SPXL were identified using gas chromatography/mass spectrometry (GC/MS) and electrospray ionization Fourier transform ion cyclotron mass spectrometry (ESI FT-ICR MS) analyses. The results show that the amount of nitrogen forms in XL is in the order of quaternary-N>pyridinic-N>pyrrolic-N, while quaternary-N in XL is easily dissolved out during thermal dissolution. In total 20 NCSs were detected in SPXL by GC/MS, and most of them are amines. Over three hundreds of NCSs were identified in SPXL by ESI FT-ICR MS, and most of them are the NCSs containing one or three nitrogen atoms. The NCSs containing one nitrogen atom are mainly dominated by N1O1, N1O2 and N1OxS1-2 class species, while most of NCSs containing three nitrogen atoms are N3OxS1-2 class species (x=1-12). The double bond equivalent (DBE) values and carbon number of the NCSs containing one nitrogen atom increase with increasing number of oxygen atoms.
-
Key words:
- lignite /
- thermal dissolution /
- nitrogen-containing species /
- carbon number /
- DBE
-
Table 1 Proximate and ultimate analyses of XL
Proximate analysis w/% Ultimate analysis wdaf/% St, d/% Mad Ad Vdaf C H N Oa 33.56 18.45 39.89 63.07 6.01 1.79 > 28.73 0.40 Mad: moisture (air dried base); Ad: ash (dry base, i.e., moisture-free base); Vdaf: volatile matter (dry and ash-free base); St, d: total sulfur (dry base); a: by difference Table 2 NCSs detected in SPXL by GC/MS
Peak Compound Molecular Formula RC /% 30 (E)-3-hydroxybenzaldehyde oxime C7H7NO2 0.48 39 4-ethyl-2, 6-dimethylpyridine C9H13N 1.52 40 4-amino-2, 3-dimethylphenol C8H11NO 0.29 43 6, 7-dihydro-1H-indol-4(5H)-one C8H9NO 0.21 45 methyl phenylcarbamodithioate C8H9NS2 0.35 47 N-benzylformamide C8H9NO 0.53 49 N-(pyridin-4-ylmethyl) ethanamine C8H12N2 0.38 51 1, 3-dimethylpyridin-2(1H)-one C7H9NO 0.71 52 Methyl benzimidate C8H9NO 0.56 54 N-phenylpropionamide C9H11NO 4.45 55 2(3H)-benzoxazolone C7H5NO2 3.34 56 benzo[d]thiazole C7H5NS 1.69 63 3-methylbenzamide C8H9NO 0.28 65 N-(1-phenylpropan-2-yl) propan-1-amine C12H19N 0.33 67 2-methyl-1, 2, 3, 4-tetrahydroquinoline C10H13N 0.62 68 3-(dimethylamino) phenol C8H11NO 0.53 69 N-benzyl-1-phenylpropan-2-amine C16H19N 1.29 70 N-phenylformamide C9H11NO 2.92 71 4-hydroxybenzamide C7H7NO2 0.29 81 isoindoline-1, 3-diimine C8H7N3 0.28 Table 3 Distribution of detected compounds in SPXL by positive-ion ESI FT-ICR MS
Number of nitrogen atoms in NCSs 0 1 2 3 4 5 6 7 8 Number of compounds 17 124 22 95 34 16 10 6 3 RC/% 7.35 28.51 7.29 25.28 11.26 6.75 9.57 1.02 2.97 Table 4 Identified N3Ox class species in SPXL by positive-ion ESI FT-IRC MS
Molecular formula DBE RC/% Molecular formula DBE RC/% N3O0 N3O3+ C15H25N3 5 0.47 C46H37N3O4 30 0.58 C18H23N3 9 2.99 C29H35N3O5 14 0.13 N3O1 C26H31N3O7 13 0.13 C18H23N3O1 9 1.28 C17H31N3O8 4 0.21 N3O2 C18H37N3O8 2 0.24 C22H27N3O2 11 0.13 C21H29N3O9 9 0.51 N3O3 C22H41N3O9 4 0.14 C12H5N3O3 12 0.46 C23H33N3O9 9 0.27 C14H23N3O3 5 0.20 C17H33N3O10 3 0.22 C21H23N3O3 12 1.45 C18H33N3O10 4 0.28 C19H35N3O10 4 0.15 C22H43N3O12 3 0.14 -
[1] KAMBARA S, TAKARADA T, TOYOSHIMA M, KATO K. Relation between functional forms of coal nitrogen and NOx emissions from pulverized coal combustion[J]. Fuel, 1995, 74(9):1247-1253. doi: 10.1016/0016-2361(95)00090-R [2] MULLINS O C, MITRA-KIRTLEY S, VAN ELP J, CRAMER S P. Molecular structure of nitrogen in coal from XANES spectroscopy[J]. Appl Spectrosc, 1993, 47(8):1268-1275. doi: 10.1366/0003702934067991 [3] YU L Y, HILDEMANN L M, NIKSA S. Characteristics of nitrogen-containing aromatic compounds in coals tars during secondary pyrolysis[J]. Fuel, 1999, 78(3):377-385. doi: 10.1016/S0016-2361(98)00130-6 [4] KELEMEN S R, GORBATY M L, KWIATEK P J. Quantification of nitrogen forms in Argonne premium coals[J]. Energy Fuels, 1994, 8(4):896-906. doi: 10.1021/ef00046a013 [5] MITRA-KIRTLEY S, MULLINS O C, VAN ELP J, CRAMER S P. Nitrogen chemical structure in petroleum asphaltene and coal by X-ray absorption spectroscopy[J]. Fuel, 1993, 72(1):133-135. doi: 10.1016/0016-2361(93)90388-I [6] ASHIDA R, MORIMOTO M, MAKINO Y, UMEMOTO S, NAKAGAWA H, MIURA K, SAITO K, KATO K. Fractional of brown coal by sequential high temperature solvent extraction[J]. Fuel, 2009, 88(8):1485-1490. doi: 10.1016/j.fuel.2008.12.003 [7] LU H Y, WEI X Y, YU R, PENG Y L, QI X Z, QIE L M, WEI Q, LV J, ZONG Z M, ZHAO W, ZHAO Y P, NI Z H, WU L. Sequential thermal dissolution of Huolinguole lignite in methanol and ethanol[J]. Energy Fuels, 2011, 25(6):2741-2745. doi: 10.1021/ef101734f [8] SHUI H F, ZHOU Y, LI H P, WANG Z C, LEI Z P, REN S B, PAN C X, WANG W W. Thermal dissolution of Shenfu coal in different solvents[J]. Fuel, 2013, 108(6):385-390. https://www.researchgate.net/publication/256712212_Thermal_dissolution_of_Shenfu_coal_in_different_solvents [9] DING M, ZHAO Y P, DOU Y Q, WEI X Y, FAN X, CAO J P, WANG Y L, ZONG Z M. Sequential extraction and thermal dissolution of Shengli lignite[J]. Fuel Process Technol, 2015, 135(7):20-24. https://www.researchgate.net/publication/276078810_Sequential_extraction_and_thermal_dissolution_of_Shengli_lignite [10] ZHAO Y P, TIAN Y J, DING M, DOU Y Q, WEI X Y, FAN X, HE X F, ZONG Z M. Difference in molecular composition of soluble organic species from two Chinese lignites with different geologic ages[J]. Fuel, 2015, 148(5):120-126. https://www.researchgate.net/publication/283536705_Difference_in_molecular_composition_of_soluble_organic_species_from_two_Chinese_lignites_with_different_geologic_ages [11] LU H, PENG P A, HSU C S. Geochemical explication of sulfur organics characterized by Fourier transform ion cyclotron resonance mass spectrometry on sulfur-rich heavy oils in Jinxian Sag, Bohai bay basin, Northern China[J]. Energy Fuels, 2013, 27(10):5861-5866. doi: 10.1021/ef4013906 [12] LI Z K, ZONG Z M, YAN H L, WANG Y G, NI H X, WEI X Y, LI Y H. Characterization of acidic species in ethanol-soluble portion from Zhaotong lignite ethanolysis by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Fuel Process Technol, 2014, 128(12):297-302. https://www.researchgate.net/publication/264560303_Characterization_of_acidic_species_in_ethanol-soluble_portion_from_Zhaotong_lignite_ethanolysis_by_negative-ion_electrospray_ionization_Fourier_transform_ion_cyclotron_resonance_mass_spectrometry [13] BAE E, NA J G, CHUNG S H, KIM H S, KIM S W. Identification of about 30000 chemical components in shale oils by electrospray ionization (ESI) and atmospheric pressure photoionization (APPI) coupled with 15 T Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) and a comparison to conventional oil[J]. Energy Fuels, 2010, 24(4):2563-2569. doi: 10.1021/ef100060b [14] HEADLEY J V, KUMAR P, DALAI A, PERU K M, BAILEY J, MCMARTIN D W, ROWLAND S M, RODGERS R P, MARSHALL A G. Fourier transform ion cyclotron resonance mass spectrometry characterization of treated Athabasca oil sands processed waters[J]. Energy Fuels, 2015, 29(5):2768-2773. doi: 10.1021/ef502007b [15] YAN H L, ZONG Z M, LI Z K, WEI X Y. Characterization of bio-oils from the alkanolyses of sweet sorghum stalk by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Fuel, 2015, 160(11):596-604. https://www.researchgate.net/publication/281210440_Characterization_of_bio-oils_from_the_alkanolyses_of_sweet_sorghum_stalk_by_electrospray_ionization_Fourier_transform_ion_cyclotron_resonance_mass_spectrometry [16] NOWICKI P, PIETRZAK R, WACHOWSKA H. X-ray photoelectron spectroscopy study of nitrogen-enriched active carbons obtained by ammoxidation and chemical activation of brown and bituminous coals[J]. Energy Fuels, 2010, 24(2):1197-1206. doi: 10.1021/ef900932g [17] GENG W H, KUMABE Y, NAKAJIMA T, TAKANASHI H, OHKI A. Analysis of hydrothermally-treated and weathered coals by X-ray photoelectron spectroscopy[J]. Fuel, 2009, 88(4):644-649. doi: 10.1016/j.fuel.2008.09.025 [18] FAN X, ZHU J L, ZHENG A L, WEI X Y, ZHAO Y P, CAO J P, ZHAO W, LU Y, CHEN L, YOU C Y. Rapid characterization of heteroatomic molecules in a bio-oil from pyrolysis of rice husk using atmospheric solids analysis probe mass spectrometry[J]. J Anal Appl Pyrolysis, 2015, 115(9):16-23. https://www.researchgate.net/publication/282625206_Rapid_characterization_of_heteroatomic_molecules_in_a_bio-oil_from_pyrolysis_of_rice_husk_using_atmospheric_solids_analysis_probe_mass_spectrometry [19] SHI Q, PAN N, LONG H Y, CUI D C, GUO X F, LONG Y H, CHUNG K H, ZHAO S Q, XU C M, HSU C S. Characterization of middle-temperature gasification coal tar. Part 3:Molecular composition of acidic compounds[J]. Energy Fuels, 2013, 27(1):108-117. doi: 10.1021/ef301431y -





 下载:
下载: