Activity and SO2 deactivation mechanism of vanadium series catalyst containing cerium
-
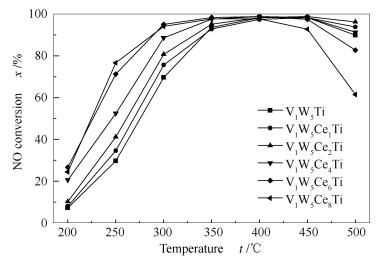
摘要: 掺杂Ce到V2O5-WO3/TiO2催化剂中,并研究其NH3-SCR脱硝性能及SO2失活机理。结果表明,V1W5Ce6Ti表现出更好的脱硝活性。采用XRD、BET、FT-IR、TG-DSC、XPS等手段表征分析Ce对催化剂性能的影响,并提出V1W5Ce6Ti硫失活机理。结果表明,Ce、V、W都能在催化剂中很好的分散,当Ce的掺杂量达到8%时,有明显的CeO2特征峰出现。在250℃时,V1W5Ti(U)表面会有NH4HSO4和(NH4)2SO4生成。掺杂Ce后,V1W5Ce6Ti催化剂的Brønste酸位和表面化学吸附氧都增加。Ce与烟气中的SO2和H2O结合生成硫酸铈盐,从而抑制硫酸铵盐的生成。同时也阻断了Ce3+与Ce4+氧化还原循环,破坏V-O-Ce结构,造成催化剂活性下降。
-
关键词:
- 选择性催化还原 /
- V2O5-WO3/TiO2 /
- 铈掺杂 /
- SO2失活
Abstract: The promotion effect of Ce modification on V2O5-WO3/TiO2 for the selective catalytic reduction (SCR) of NOx with NH3 and the SO2 deactivation mechanism were investigated. Compared with V1W5Ti catalyst, the advantage of V1W5Ce6Ti catalyst shows a good catalytic activity. These catalysts were investigated by means of XRD, BET, FT-IR, TG-DSC and XPS. The results demonstrate that the active components of V and W are well-dispersed, while a small cluster of cubic CeO2 appears over the V1W5Ce8Ti catalyst. The sulfation of V1W5Ti under reactive conditions can generate NH4HSO4 and (NH4)2SO4 at 250℃. The Ce additive to V1W5Ti could provide stronger Brønsted acid sites and more chemisorbed oxygen. The deposited ammonium sulfate on V1W5Ce6Ti catalyst is much smaller than that on V1W5Ti because the cerium sulfates species on the surface of V1W5Ce6Ti is formed and the deposition of ammonium sulfate is inhibited, which can disrupt the redox cycle between Ce3+ and Ce4+ and break the V-O-Ce structure, causing the deactivation of V1W5Ce6Ti catalyst.-
Key words:
- selective catalytic reduction /
- V2O5-WO3/TiO2 /
- cerium doped /
- SO2 deactivation
-
表 1 不同催化剂的比表面积、总孔容和平均孔径
Table 1 BET surface area, total pore volume, average pore diameter of different catalysts
Sample BET surface area A/(m2·g-1) Total pore volume v/(cm3·g-1) Average pore diameter d/nm V1W5Ti 86.1 0.315 12.9 V1W5Ce2Ti 76.8 0.312 9.8 V1W5Ce6Ti 72.3 0.282 9.9 V1W5Ce8Ti 70.4 0.276 9.9 表 2 不同催化剂的表面原子浓度
Table 2 Surface atom percentages of different catalysts determined by XPS
Sample Surface atom concentration wmol/% V W Ti O Ce S V1W5Ti 1.98 5.78 20.78 71.46 - - V1W5Ce6Ti 1.67 5.35 18.82 73.79 0.36 - V1W5Ce6Ti (S) 1.74 2.53 20.33 74.11 0.21 1.08 -
[1] ZHANG L, LI L, CAO Y, YAO X, GE C, GAO F, DENG Y, TANG C, DONG L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3[J]. Appl Catal B:Environ, 2015, 165:589-598. doi: 10.1016/j.apcatb.2014.10.029 [2] KONG M, LIU Q C, ZHU B H, YANG J, LI L, ZHOU Q, REN S. Synergy of KCl and Hg-el on selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts[J]. Chem Eng J, 2015, 264:815-823. doi: 10.1016/j.cej.2014.12.038 [3] JIN R, LIU Y, WANG Y, CEN W, WU Z, WANG H, WENG X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature[J].Appl Catal B:Environ, 2014, 148:582-588. [4] BUSCA G, LIETTI L, RAMIS G, BERTI F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts:A review[J]. Appl Catal B:Environ, 1998, 18(1):1-36. [5] OLIVERI G, BUSCA G, LORENZELLI V, Structure and surface-area evolution of vanadia-on-titania powders upon heat-treatment[J]. Mater Chem Phys, 1989, 22(5):511-521. doi: 10.1016/0254-0584(89)90063-1 [6] AMORES J M G, ESCRIBANO V S, BUSCA G. Anatase crystal-growth and phase-transformation to rutile in high-area TiO2, MoO3-TiO2 and other TiO2-supported oxide catalytic-systems[J]. J Mater Chem, 1995, 5(8):1245-1249. doi: 10.1039/JM9950501245 [7] CHEN J P, BUZANOWSKI M A, YANG R.T, CICHANOWICZ J E. Deactivation of the vanadia catalyst in the selective catalytic reduction process[J]. J Air Waste Manage, 1990, 40(10):1403-1409. doi: 10.1080/10473289.1990.10466793 [8] JIANG Y, XING Z, WANG X, ET A L. Activity and characterization of a Ce-W-Ti oxide catalyst prepared by a single step sol-gel method for selective catalytic reduction of NO with NH3[J]. Fuel, 2015, 151:124-129. doi: 10.1016/j.fuel.2015.01.061 [9] LEE K J, KUMAR P A, MAQBOOL M S. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR:Physico-chemical properties and catalytic activity[J]. Appl Catal B:Environ, 2013, 142:705-717. [10] KWON D W, NAM K B, HONG S C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3[J]. Appl Catal B:Environ, 2015, 166-167:37-44. doi: 10.1016/j.apcatb.2014.11.004 [11] 刘昕, 宁平, 李昊, 宋忠贤, 王燕彩, 张金辉, 唐小苏, 王明智, 张秋林.水相法制备Ce-W@TiO2催化剂的氨选择性催化还原NO (NH3-SCR) 活性和抗SO2性能研究[J].燃料化学学报, 2016, 44(2):225-231. doi: 10.1016/S1872-5813(16)30010-XLIU Xin, NING Ping, LI Hao, SONG Zhong-xian, WANG Yan-cai, ZHANG Jin-hui, TANG Xiao-su, WANG Ming-zhi, ZHANG Qiu-lin. Probing NH3-SCR catalytic activity and SO2 resistance over aqueous-phase synthesized Ce-W@TiO2 catalyst[J]. J Fuel Chem Technol, 2016, 44(2):225-231. doi: 10.1016/S1872-5813(16)30010-X [12] KWON D W, PARK K H, HONG S C. Enhancement of SCR activity and SO2 resistance on VOx/TiO2 catalyst by addition of molybdenum[J]. Chem Eng J, 2016, 284:315-324. doi: 10.1016/j.cej.2015.08.152 [13] ZHANG L, LI L, CAO Y. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3[J]. Appl Catal B:Environ, 2015, 165:589-598. doi: 10.1016/j.apcatb.2014.10.029 [14] KONG M, LIU Q, WANG X, REN S, YANG J, ZHAO D, XI W, YAO L. Performance impact and poisoning mechanism of arsenic over commercial V2O5-WO3/TiO2 SCR catalyst[J]. Catal Commun, 2015, 72:121-126. doi: 10.1016/j.catcom.2015.09.029 [15] ZHAO H, BENNICI S, SHEN J, AUROUX A. The influence of the preparation method on the structural, acidic and redox properties of V2O5-TiO2/SO42- catalysts[J]. Appl Catal A:Gen, 2009, 356(2):121-128. doi: 10.1016/j.apcata.2008.12.037 [16] STOILOVA D, GEORGIEV M, MARINOVA D.Infrared study of the vibrational behavior of CrO42- guest ions matrix-isolated in metal (Ⅱ) sulfates (Me=Ca, Sr, Ba, Pb)[J]. J Mol Struct, 2005, 738(1):211-215. https://www.researchgate.net/publication/244287018_Infrared_study_of_the_vibrational_behavior_of_CrO_4_2-_guest_ions_matrix-isolated_in_metal_Ⅱ_sulfates_MeCa_Sr_Ba_Pb [17] TOPSØE N Y, DUMESIC J A, TOPSØE H.Vanadia/titania catalysts for selective catalytic reduction (SCR) of nitric oxide by ammonia. Ⅱ. Studies of active sites and formulation of catalytic cycles[J]. J Catal, 1995, 151(1):241-252. doi: 10.1006/jcat.1995.1025 [18] GUO XY, BARTHOLOMEW C, HECKER W, BAXTER LL. Effects of sulfate species on V2O5/TiO2 SCR catalysts in coal and biomass-fired systems[J]. Appl Catal B:Environ, 2009, 92(1):30-40. [19] MA Z, WU XD, FENG Y, SI ZC, WENG D, SHI L. Low-temperature SCR activity and SO2 deactivation mechanism of Ce-modified V2O5-WO3/TiO2 catalyst[J]. Prog Nat Sci Mater, 2015, 25(4):342-352. doi: 10.1016/j.pnsc.2015.07.002 [20] YANG J, YANG Q, SUN J. Effects of mercury oxidation on V2O5-WO3/TiO2 catalyst properties in NH3-SCR process[J]. Catal Commun, 2015, 59:78-82. doi: 10.1016/j.catcom.2014.09.049 [21] KOHIKI S, SHIMOOKA H, TAKADA S, SHIMIZU A, HIRAKAWA T, TAKAHASHI S. Synthesis and magnetic properties of mesoporous vanadium oxide sulphate[J]. Chem Lett, 2002, 7:670-671. [22] LIU CX, CHEN L, LI JH, MA L, ARANDIYAN H, DU Y. Enhancement of activity and sulfur resistance of CeO2 supported on TiO2-SiO2 for the selective catalytic reduction of NO by NH3[J]. Environ Sci Technol, 2012, 46(11):6182-6189. doi: 10.1021/es3001773 -





 下载:
下载: