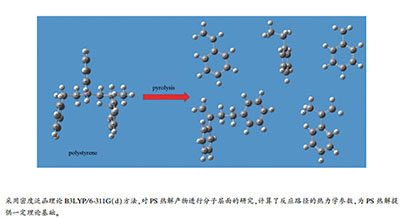
| [1] |
邓亮, 徐海萍, 谢华清.废旧聚苯乙烯塑料再生再利用技术现状[J].上海第二工业大学学报, 2014, (2):114-120. doi: 10.3969/j.issn.1001-4543.2014.02.006DENG Liang, XU Hai-ping, XIE Hua-qing. Research on techniques of waste polystyrene plastics recycling and reusing[J]. J Shanghai Doly Univ, 2014, (2):114-120. doi: 10.3969/j.issn.1001-4543.2014.02.006
|
| [2] |
RUTKOWSKI P, KUBACKI A. Influence of polystyrene addition to cellulose on chemical structure and properties of bio-oil obtained during pyrolysis[J]. Energy Convers Manage, 2006, 47(6):716-731. doi: 10.1016/j.enconman.2005.05.017
|
| [3] |
ARTETXE M, LOPEZ G, AMUTIO M. Styrene recovery from polystyrene by flash pyrolysis in a conical spouted bed reactor[J]. Waste Manag, 2015, 45(1):26-33. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=49c131d0d1a0ee71ec92ad1d0878b815
|
| [4] |
李春华.聚苯乙烯降解过程研究[D].天津: 天津大学, 2006.LI Chun-hua. Study on the degradation of polystyrene[D]. Tianjin: Tianjin University, 2006.
|
| [5] |
刘贤响, 尹笃林, 黄文质.废聚苯乙烯的热裂解与催化裂解[J].石油化工, 2009, 38(2):189-192. doi: 10.3321/j.issn:1000-8144.2009.02.015LIU Xian-xiang, YIN Du-lin, HUANG Wen-zhi. Catalytic cracking and thermal cracking of waste polystyrene[J]. Petrochem Technol, 2009, 38(2):189-192. doi: 10.3321/j.issn:1000-8144.2009.02.015
|
| [6] |
FERNANDES L, GASPAR H, BERNARDO G. Inhibition of thermal degradation of polystyrene by C60 and PCBM:A comparative study[J]. Polym Test, 2014, 40(6):3-9.
|
| [7] |
PRATHIBA R, SHRUTHI M, MIRANDA L R. Pyrolysis of polystyrene waste in the presence of activated carbon in conventional and microwave heating using modified thermocouple[J]. Waste Manag, 2018, 76:528-536. doi: 10.1016/j.wasman.2018.03.029
|
| [8] |
杨震, 高松亭.废聚苯乙烯塑料热降解回收苯乙烯单体的研究[J].环境科学, 1997, (2):45-47+95-96. doi: 10.3321/j.issn:1001-6929.1997.02.016YANG ZHEN, GAO Song-ting. Study on thermal degradation recovery of styrene monomer from waste polystyrene plastics[J]. Environ Chem, 1997, (2):45-47+95-96. doi: 10.3321/j.issn:1001-6929.1997.02.016
|
| [9] |
SHAH J, JAN M R, ADNAN. Metal decorated montmorillonite as a catalyst for the degradation of polystyrene[J]. J Taiwan Inst Chem Eng, 2017. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ae54cb27b88aeffb7c9780b7b6134845
|
| [10] |
张敏华, 李春华, 姜浩锡.热重质谱法研究聚苯乙烯热降解机理[J].化工进展, 2008, 27(4):609-612. doi: 10.3321/j.issn:1000-6613.2008.04.029ZHANG Min-hua, LI Chun-hua, JIANG Hao-xi. Thermal degradation mechanism of polystyrene by TG-MS[J]. Chem Ind Eng Prog, 2008, 27(4):609-612. doi: 10.3321/j.issn:1000-6613.2008.04.029
|
| [11] |
朱新生, 戴建平, 李引擎.聚苯乙烯热降解动力学参数与降解反应机理关系[J].火灾科学, 2001, 10(1):24-28. doi: 10.3969/j.issn.1004-5309.2001.01.004ZHU Xin-sheng, DAI Jian-ping, LI Yin-qing. Relationship between thermal degradation mechanism and kineticp parameters of polystyrene[J]. Fire Safe Sci, 2001, 10(1):24-28. doi: 10.3969/j.issn.1004-5309.2001.01.004
|
| [12] |
FONDO M, OCAMPO N, GARC A-DEIBE A M. Discovering the complex chemistry of a simple Ni(Ⅱ)/H(3)L system:Magnetostructural characterization and DFT calculations of Di-and polynuclear nickel(Ⅱ) compounds[J]. Inorg Chem, 2009, 48(20):9861-9873. doi: 10.1021/ic9014916
|
| [13] |
王国成.等规聚苯乙烯和乙烯-苯乙烯共聚物的合成、表征及结晶性能研究[D].杭州: 浙江大学, 2005. http://www.doc88.com/p-1184324207389.htmlWANH Guo-cheng. Synthesis, characterization and crystallization properties of isometric polystyrene and ethylene-styrene polymers[J]. Hangzhou: Zhejiang University, 2005. http://www.doc88.com/p-1184324207389.html
|
| [14] |
彭学成.无规聚苯乙烯的物理老化研究进展[J].齐鲁石油化工, 2007, 35(2):137-140. doi: 10.3969/j.issn.1009-9859.2007.02.017PENG Xue-cheng. Development advances in physical aging of atatic polystyrene[J]. Qilu Pet Technol, 2007, 35(2):137-140. doi: 10.3969/j.issn.1009-9859.2007.02.017
|
| [15] |
LAGASSE R R, MAXWELL B. An experimental study of the kinetics of polymer crystallization during shear flow[J]. Polym Eng Sci, 2010, 16(3):189-199.
|
| [16] |
BARTON B D, STEIN S E. Pyrolysis of alkyl benzenes. Relative stabilities of methyl-substituted benzyl radicals[J]. Chem Inf, 1980, 11(46):2141-2145.
|
| [17] |
AND M M, GOLDEN D M. Hydrocarbon bond dissociation energies[J]. Annu Rev Phys Chem, 1982, 33(33):493-532. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0220799363/
|
| [18] |
周俊波.典型通用乙烯基塑料在二次反应最小化条件下的热解实验研究[D].武汉: 华中科技大学, 2016.ZHOU Jun-bo. Experimental study on pyrolysis of typical universal vinyl plastics under the condition of secondary reaction minimization[D].Wuhan: Huazhong University of Science and Technology, 2016.
|
| [19] |
POUTSMA M L. Further considerations of the sources of the volatiles from pyrolysis of polystyrene[J]. Polym Degrad Stab, 2009, 94(11):2055-2064. doi: 10.1016/j.polymdegradstab.2009.07.011
|
| [20] |
KIM S S, KIM S. Pyrolysis characteristics of polystyrene and polypropylene in a stirred batch reactor[J]. Chem Eng J, 2004, 98(1):53-60. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3c4cef55872eb0bb30867f93e606ff01
|
| [21] |
LEHRLE R S, ATKINSON D J, BATE D M. Diagnosing mechanisms of oligomer formation in the thermal degradation of polymers[J]. Polym Degrad Stab, 1996, 52(2):183-196. doi: 10.1016/0141-3910(96)00014-6
|
| [22] |
MCNEILL I C, ZULFIQAR M, KOUSAR T. A detailed investigation of the products of the thermal degradation of polystyrene[J]. Polym Degrad Stab, 1990, 28(2):131-151. doi: 10.1016/0141-3910(90)90002-O
|





 下载:
下载: