Selective oxidation of methanol to methyl formate over bimetallic Au-Pd nanoparticles supported on SiO2
-
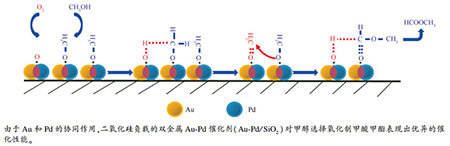
摘要: 甲醇选择氧化制备甲酸甲酯(MF)是延伸甲醇产业链、开发高附加值下游产品的有效途径之一,负载型Au及Pd催化剂在这一反应中表现出优异的低温催化性能。为探索实用、高效和易再生的甲醇选择氧化催化剂,同时揭示双金属颗粒中Au和Pd的协同效应及甲醇氧化反应机理,本研究制备了一系列二氧化硅负载的Au-Pd催化剂(Au-Pd/SiO2),详细研究了其对甲醇选择氧化制甲酸甲酯的催化性能。结果表明,Au和Pd总负载量为0.6%、且Au/Pd质量比为2时,所制备的Au2-Pd1/SiO2催化剂表现出优异的甲醇氧化催化性能;在130℃下,甲醇转化率达到57.0%,MF选择性为72.7%。多种表征结果显示,Au-Pd双金属纳米颗粒粒径为2-4 nm,高度分散于SiO2载体表面,倾向于生成孪晶结构并暴露(111)晶面,这些因素是Au-Pd/SiO2具有优异催化性能的主要原因。通过DRIFTS表征研究,提出了一个可能的MF生成机理:即甲醇首先与处于Au-Pd纳米粒子界面的表面氧作用,生成化学吸附的甲氧基;随后,甲氧基经去质子作用生成吸附的甲醛物种,后者与相邻的甲氧基物种亲核反应,并经β-H消除后得到目标产物MF。Abstract: Selective oxidation of methanol to methyl formate (MF) is one of the most attractive processes to get valuable methanol-downstream products, where the supported Au and Pd catalysts were proved rather effective at low temperature. To search for highly active, regenerable and practical catalysts as well as to reveal the synergy of Au-Pd and reaction mechanism for the methanol oxidation, a series of silica supported Au-Pd nanoparticles (Au-Pd/SiO2) were prepared and their catalytic performance in the oxidation of methanol to MF with molecular oxygen was investigated in this work. The results indicate that the Au2-Pd1/SiO2 catalyst with an Au+Pd loading of only 0.6% and a Au/Pd mass ratio of 2 exhibits excellent performance in the methanol oxidation with oxygen; the conversion of methanol over Au2-Pd1/SiO2 reaches 57.0% at 130℃, with a selectivity of 72.7% to MF. Various characterization results illustrate that the Au-Pd bimetallic nanoparticles (2-4 nm) are highly dispersed on the silica surface, inclined to take a twinned structure and present the (111) planes, which may contribute to the high activity of Au-Pd/SiO2 in the oxidation of methanol to MF. A possible reaction mechanism was proposed on the basis of DRIFTS results:methanol was first activated by surface oxygen on the interface of Au-Pd nanoparticles, forming the chemisorbed methoxy species; the methoxy species was then deprotonated to adsorbed formaldehyde species, which reacted with another methoxy species, producing MF by nucleophilic attack and subsequent β-H elimination.
-
Key words:
- selective oxidation of methanol /
- methyl formate /
- gold /
- palladium /
- silica /
- bimetallic nanoparticles
-
Table 1 Au and Pd loadings and surface areas of various Au-Pd/SiO2 catalysts
Catalyst Au loading w/% Pd loading w/% ABET/(m2·g-1) SiO2 - - 209 Au/SiO2 0.53 - 196 Au2-Pd1/SiO2 0.40 0.20 198 Au1-Pd1/SiO2 0.28 0.29 202 Au1-Pd2/SiO2 0.18 0.38 194 Pd/SiO2 - 0.55 204 Table 2 Catalytic performance of various Au-Pd/SiO2 catalysts in methanol oxidation
Catalyst Temperature t/℃ Methanol conversion x/% Product selectivity s/% MF CO2 Au/SiO2 110 10.9 100 0 130 17.1 100 0 150 29.2 100 0 Au2-Pd1/SiO2 110 32.3 90.2 9.8 130 57.0 72.7 27.3 150 63.5 5.2 94.8 Au1-Pd1/SiO2 70 18.3 100 0 90 23.2 100 0 110 82.0 0 100 Au1-Pd2/SiO2 70 10.3 100 0 90 23.9 90.1 9.9 110 88.4 0 100 Pd/SiO2 110 13.5 93.6 6.4 130 32.1 66.6 33.4 150 100 0 100 Au1-Ag1/SiO2 150 7.3 100 0 170 31.3 81.8 18.2 Au1-Cu1/SiO2 150 10.4 100 0 170 16.0 100 0 -
[1] GEÂRARD E, GOÈTZ H, PELLEGRINI S, CASTANET Y, MORTREUX A. Epoxide-tertiary amine combinations as efficient catalysts for methanol carbonylation into methyl formate in the presence of carbon dioxide[J]. Appl Catal A:Gen, 1998, 170:297-306. doi: 10.1016/S0926-860X(98)00060-X [2] LI N, WANG S B, SUN YH, LI S G. First principles studies on the selectivity of dimethoxymethane and methyl formate in methanol oxidation over V2O5/TiO2-based catalysts[J]. Phys Chem Chem Phys, 2017, 19:19393-19406. doi: 10.1039/C7CP02326J [3] KAICHEV V V, POPOVA G YA, CHESALOV YU A, SARAEV A A, ZEMLYANOV D Y, BELOSHAPKIN S A, KNOP-GERICKE A, SCHLÖGL R, ANDRUSHKEVICH T V, BUKHTIYAROV V I. Selective oxidation of methanol to form dimethoxymethane and methyl formate over a monolayer V2O5/TiO2 catalyst[J]. J Catal, 2014, 311:59-70. doi: 10.1016/j.jcat.2013.10.026 [4] ZHAO Y B, QIN Z F, WANG G F, DONG M, HUANG L C, WU Z W, FAN W B, WANG J G. Catalytic performance of V2O5/ZrO2-Al2O3 for methanol oxidation[J]. Fuel, 2013, 104:22-27. doi: 10.1016/j.fuel.2010.03.008 [5] LI W Z, LIU H C, IGLESIA E. Structures and properties of zirconia-supported ruthenium oxide catalysts for the selective oxidation of methanol to methyl formate[J]. J Phys Chem B, 2006, 110:23337-23342. doi: 10.1021/jp0648689 [6] AI M. The production of methyl formate by the vapor-phase oxidation of methanol[J]. J Catal, 1982, 77:279-288. doi: 10.1016/0021-9517(82)90168-3 [7] LIU G B, ZHANG Q D, HAN Y Z, TSUBAKI N, TAN Y S. Low-temperature oxidation of dimethyl ether to methyl formate with high selectivity over MoO3-SnO2 catalysts[J]. J Fuel Chem Technol, 2013, 41(2):223-227. doi: 10.1016/S1872-5813(13)60014-6 [8] LIU J L, ZHAN E S, CAI W J, LI J, SHEN W J. Methanol selective oxidation to methyl formate over ReOx/CeO2 catalysts[J]. Catal Lett, 2008, 120(3/4):274-280. [9] LIU H C, IGLESIA E. Effects of support on bifunctional methanol oxidation pathways catalyzed by polyoxometallate Keggin clusters[J]. J Catal, 2004, 223(1):16l-169. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b44f334e34e211dd3b2e78f6c86c101e [10] WOJCIESZAK R, GHAZZAL M N, GAIGNEAUX E M, RUIZ P. Oxidation of methanol to methyl formate over supported Pd nanoparticles:Insights into the reaction mechanism at low temperature[J]. Catal Sci Technol, 2014, 4(9):3298-3305. doi: 10.1039/C4CY00531G [11] WANG R Y, WU Z W, CHEN C M, QIN Z F, ZHU H Q, WANG G F, WANG H, WU C M, DONG W W, FAN W B, WANG J G. Graphene-supported Au-Pd bimetallic nanoparticles with excellent catalytic performance in selective oxidation of methanol to methyl formate[J]. Chem Commun, 2013, 49(74):8250-8252. doi: 10.1039/c3cc43948h [12] WHITING G T, KONDRAT S A, HAMMOND C, DIMITRATOS N, HE Q, MORGAN D J, DUMMER N F, BARTLEY J K, KIELY C J, TAYLOR S H, HUTCHINGS G J. Methyl formate formation from methanol oxidation using supported gold-palladium nanoparticles[J]. ACS Catal, 2015, 5:637-644. doi: 10.1021/cs501728r [13] CHEN Q B, LUO L T. Effects of reductant on catalytic performance of Au-Pd/CeO2 catalysts for partial oxidation of methanol[J]. J Fuel Chem Technol, 2008, 36(3):332-337. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb200803015 [14] BAKER T A, LIU X, FRIEND C M. The mystery of gold's chemical activity:Local bonding, morphology and reactivity of atomic oxygen[J]. Phys Chem Chem Phys, 2010, 13(1):34-46. [15] ZHANG Q F, LI Y K, LI Z, LI C, YE L, YONG L. Structured nanoporous-gold/Al-fiber:Galvanic deposition preparation and reactivity for the oxidative coupling of methanol to methyl formate[J]. Green Chem, 2014, 16(6):2992-2996. doi: 10.1039/C3GC42561D [16] WITTSTOCK A, ZIELASEK V, BIENER J, FRIEND C M, BÄUMER M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature[J]. Science, 2010, 327(5963):319-322. doi: 10.1126/science.1183591 [17] LU D, ZHANG Y, LIN S, WANG L, WANG C. Synthesis of Pt-Au bimetallic nanoparticles on graphene-carbon nanotube hybrid nanomaterials for nonenzymatic hydrogen peroxide sensor[J]. Talanta, 2013, 112(15):111-116. [18] WANG R Y, WU Z W, WANG G F, QIN Z F, CHEN C M, DONG M, ZHU H Q, FAN W B, WANG J G. Highly active Au-Pd nanoparticles supported on three-dimensional graphene-carbon nanotube hybrid for selective oxidation of methanol to methyl formate[J]. RSC Adv, 2015, 5(56):44835-44839. doi: 10.1039/C5RA06025G [19] XU J, WHITE T, LI P, HE C H, YU J G, YUAN W K, HAN Y F. Biphasic Pd-Au alloy catalyst for low-temperature CO oxidation[J]. J Am Chem Soc, 2010, 132(30):10398-10406. doi: 10.1021/ja102617r [20] TAN L F, CHEN D, LIU H Y, TANG F Q. A silica nanorattle with a mesoporous shell:An ideal nanoreactor for the preparation of tunable gold cores[J]. Adv Mater, 2010, 22(43):4885-4889. doi: 10.1002/adma.201002277 [21] WANG A Q, CHANG C M, MOU C Y. Evolution of catalytic activity of Au-Ag bimetallic nanoparticles on mesoporous support for CO oxidation[J]. J Phys Chem B, 2005, 109(40):18860-18867. doi: 10.1021/jp051530q [22] LU C L, PRASAD K S, WU H L, HO J A, HUANG M H. Au nanocube-directed fabrication of Au-Pd core-shell nanocrystals with tetrahexahedral, concave octahedral, and octahedral structures and their electrocatalytic activity[J]. J Am Chem Soc, 2010, 132(41):14546-14553. doi: 10.1021/ja105401p [23] XU J G, WILSON A R, RATHMELL A R, HOWE J, CHI M F, WILEY B J. Synthesis and catalytic properties of Au-Pd nanoflowers[J]. Acs Nano, 2011, 5(8):6119-6127. doi: 10.1021/nn201161m [24] BULUSHEV D A, YURANOV I, SUVOROVA E I, BUFFAT P A, KIWIMINSKER L. Highly dispersed gold on activated carbon fibers for low-temperature CO oxidation[J]. J Catal, 2004, 224(1):8-17. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2c1ab8c29b5f884e25513eba0f8e703d [25] LIU R, YU Y, YOSHIDA K, LI G, JIANG H, ZHANG M, ZHAO F, FUJITA S, ARAI M. Physically and chemically mixed TiO2-supported Pd and Au catalysts:Unexpected synergistic effects on selective hydrogenation of citral in supercritical CO2[J]. J Catal, 2010, 269(1):191-200. [26] PRITCHARD J, KESAVAN L, PICCININI M, HE Q, TIRUVALAM R, DIMITRATOS N, LOPEZ-SANCHEZ J A, CARLEY A F, EDWARDS J K, KIELY C J, HUTCHINGS G J. Direct synthesis of hydrogen peroxide and benzyl alcohol oxidation using Au-Pd catalysts prepared by sol immobilization[J]. Langmuir, 2010, 26(21):16568-16577. doi: 10.1021/la101597q [27] HSU C, HUANG C, HAO Y, LIU F. Au/Pd core-shell nanoparticles for enhanced electrocatalytic activity and durability[J]. Electrochem Commun, 2012, 23(1):133-136. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3fe86b998810eb29d8afa18038131899 [28] GUO X N, BRAULT P, ZHI G J, CAILLARD A, JIN G Q, COUTANCEAU C, BARANTON S, GUO X Y. Synergistic combination of plasma sputtered Pd-Au bimetallic nanoparticles for catalytic methane combustion[J]. J Phys Chem C, 2011, 115(22):11240-11246. doi: 10.1021/jp203351p [29] ZHANG G J, WANG Y E, X WANG, CHEN Y, ZHOU Y, TANG Y, LU L, BAO J, LU T. Preparation of Pd-Au/C catalysts with different alloying degree and their electrocatalytic performance for formic acid oxidation[J]. Appl Catal B:Environ, 2011, 102(3):614-619. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=98a67f8bd5cfe38ad5e495c0d249ff9c [30] CZELEJ K, CWIEKA K, COLMENARES J C, KURZYDLOWSKI K J, XU Y. Toward a comprehensive understanding of enhanced photocatalytic activity of the bimetallic PdAu/TiO2 catalyst for selective oxidation of methanol to methyl formate[J]. ACS Appl Mater Interfaces, 2017, 9:31825-31833. doi: 10.1021/acsami.7b08158 [31] KOMINAMI H, SUGAHARA H, HASHIMOTO K. Photocatalytic selective oxidation of methanol to methyl formate in gas phase over titanium(Ⅳ) oxide in a flow-type reactor[J]. Catal Commun, 2010, 11(5):426-429. doi: 10.1016/j.catcom.2009.11.014 [32] WOJCIESZAK R, MATEOS-BLANCO R, HAUWAERT D, CARRAZAN S R G, GAIGNEAUX E AND, RUIZ P. Influence of the preparation method on catalytic properties of Pd/TiO2 catalysts in the reaction of partial oxidation of methanol[J]. Curr Catal, 2013, 2:27-34. doi: 10.2174/2211544711302010006 [33] YAN C, DONG Q N, REN J, SUN Y H. Studies on mechanism of methanol decomposition over Pd/CeO2 catalyst[J]. Chem J Chin Univ, 2002, 23(12):2329-2331. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gdxxhxxb200212021 [34] BONURA G, CORDARO M, SPADARO L, CANNILLA C, ARENA F, FRUSTERI F. Hybrid Cu-ZnO-ZrO2/H-ZSM-5 system for the direct synthesis of DME by CO2 hydrogenation[J]. Appl Catal B:Environ, 2013, 140-141:16-24. doi: 10.1016/j.apcatb.2013.03.048 [35] HANAOKA T, HATSUTA T, TAGO T, KISHIDAAND M, WAKABAYASHI K. Control of the rhodium particle size of the silica-supported catalysts by using microemulsion[J]. Appl Catal A:Gen, 2000, 190(1/2):291-296. [36] LOCHAŘ V. FT-IR study of methanol, formaldehyde and methyl formate adsorption on the surface of Mo/Sn oxide catalyst[J]. Appl Catal A:Gen, 2006, 309(1):33-36. doi: 10.1016/j.apcata.2006.04.030 [37] LOCHAŘ V, MACHEK J, TICHY J. Mechanism of selective oxidation of methanol over stannic oxide-molybdenum oxide catalyst[J]. Appl Catal A:Gen, 2002, 228(1):95-101. [38] BURCHAM L J, BADLANI M, WACHS I E. The origin of the ligand effect in metal oxide catalysts:Novel fixed-bed in situ infrared and kinetic studies during methanol oxidation[J]. J Catal, 2001, 203(1):104-121. doi: 10.1006/jcat.2001.3312 [39] LIU X Y, MADIX R J, FRIEND C M. Unraveling molecular transformations on surfaces:A critical comparison of oxidation reactions on coinage metals[J]. Chem Soc Rev, 2008, 37(10):2243-2261. doi: 10.1039/b800309m [40] XU B, LIU X, HAUBRICH J, HAUBRICH J, FRIEND C M. Vapour-phase gold-surface-mediated coupling of aldehydes with methanol[J]. Nat Chem, 2010, 2(1):61-65. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e767bdddb079db82c9070bcb0234379e -





 下载:
下载: