-
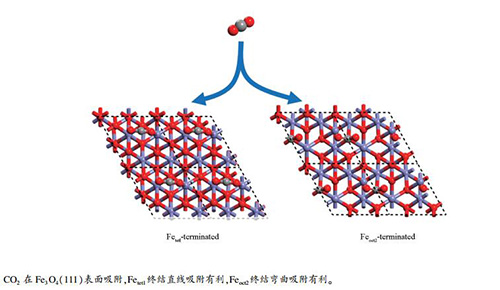
摘要: 利用密度泛函理论研究了CO2在Fe3O4(111)表面Fetet1和Feoct2两种终结的吸附行为。在Fetet1终结表面,当覆盖度为1/5 ML时,CO2倾向于线性吸附;而在高覆盖度下,弯曲的CO2与表面O作用形成CO32-结构。在Feoct2终结表面,CO2倾向于弯曲吸附,在1/6 ML和1/3 ML覆盖度时都可以形成CO32-和-COO结构。覆盖度对Fetet1终结的表面影响很弱,但是对Feoct2终结的表面影响很大。从热力学上来说,CO2在Feoct2终结表面的吸附要比Fetet1终结表面更有利。Abstract: Density functional theory calculations were used to investigate CO2 adsorption behaviors on Fetet1-and Feoct2-terminated surface of Fe3O4 (111). The results indicated that on the Fetet1-terminated surface, the linear CO2 is favored at 1/5 monolayer (ML), whereas the bent CO2 bonded to surface O, i.e. carbonate structure, becomes possible at higher coverage. On the Feoct2-terminated surface, the bent CO2 is favored; both carbonate and carboxylate structure are formed at both 1/6 and 1/3 ML. Meanwhile, the Fetet1-terminated Fe3O4(111) surface has weak coverage effects, whereas the Feoct2-terminated Fe3O4(111) surface has strong coverage effects; the Feoct2-terminated surface is thermodynamically more favorable than the Fetet1-terminated surface for CO2 adsorption.
-
Key words:
- Fe3O4 /
- density functional theory /
- CO2 /
- adsorption
-
Figure 5 Local density of states of adsorbed CO2 on Fe3O4(111) surfaces (a)/(b): Figure 2(b)/2(c) on Fetet1-terminated surface; (c)/(d): Figure 3(c)/ Figure 4(a) on Feoct2-terminated surface
(solid lines, after adsorption; dotted lines, before adsorption; red and blue lines, the front and back of two co-chemisorbed CO2 molecules on the surface)
Table 1 Computed net charges (q) of CO2 on the Fetet1- and Feoct2-terminated Fe3O4(111) surface
Fe3O4 surface Adsorption model ML OCO qC qO(1) qO(2) qCO2 None Free CO2 180.0 1.02 -0.51 -0.51 0 Fetet1-terminated surface 2(a) 1/5 180.0 0.95 -0.51 -0.41 0.03 2(b) 1/5 177.8 0.95 -0.50 -0.44 0.01 2(c) 2/5a 178.1 0.98 -0.51 -0.42 0.05 2/5b 131.4 0.73 -0.56 -0.55 -0.38 Feoct2-terminated surface 3(a) 1/6 179.7 0.94 -0.53 -0.46 -0.05 3(b) 1/6 146.4 0.46 -0.50 -0.47 -0.51 3(c) 1/6 126.3 0.72 -0.58 -0.58 -0.44 3(d) 1/6 127.5 0.71 -0.59 -0.56 -0.44 3(e) 1/6 141.9 0.39 -0.52 -0.47 -0.60 3(f) 1/6 126.1 0.66 -0.57 -0.56 -0.47 4(1) 1/3a 146.0 0.50 -0.49 -0.42 -0.41 1/3b 128.1 0.70 -0.59 -0.55 -0.44 4(b) 1/3a 147.6 0.51 -0.48 -0.43 -0.40 1/3b 127.7 0.70 -0.56 -0.59 -0.45 note: a and b represent the front and back of two co-chemisorbed CO2 molecules on the surface model, respectively -
[1] GEUS W J. Preparation and properties of iron oxide and metallic iron catalysts[J]. Appl Catal A:Gen, 1986, 25(1/2):313-333. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0230036199 [2] RAO K R, HUGGINS F E, MAHAJAN V, HUFFMAN G P. Mossbauer spectroscopy study of iron-based catalysts used in Fischer-Tropsch synthesis[J]. Top Catal, 1995, 2(1/4):71-78. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=CC026298908 [3] ZHANG H B, SCHRADER G L. Characterization of a fused iron catalyst for Fischer-Tropsch synthesis by in situ laser raman spectroscopy[J]. J Catal, 1985, 95(1):325-332. doi: 10.1016/0021-9517(85)90038-7 [4] RETHWISCH D G, DUMESIC J A. Adsorptive and catalytic properties of supported metal oxides Ⅲ. Water-gas shift over supported iron and zinc oxides[J]. J Catal, 1986, 101(1):35-42. doi: 10.1016/0021-9517(86)90226-5 [5] HUANG C S, XU L G, DAVIS B H. Fishcher-Tropsch synthesis:Impact of pretreatment of ultrafine iron oxide upon catalyst structure and selectivity[J]. Fuel Sci Technol Int, 1993, 11(5/6):639-664. https://www.researchgate.net/publication/232855235_Fischer-tropsch_synthesis_impact_of_pretreatment_of_ultrafine_iron_oxide_upon_catalyst_structure_and_selectivity [6] NEWSOME D S. The water-gas shift reaction[J]. Catal Rev Sci Eng, 1980, 21(2):275-318. doi: 10.1080/03602458008067535 [7] ZHANG C L, LI S, WANG L J, WU T H, PENG S Y. Studies on the decomposition of carbon dioxide into carbon with oxygen-deficient magnetite I. Preparation, characterization of magnetite, and its activity of decomposing carbon dioxide[J]. Mater Chem Phys, 2000, 62(1):44-51. doi: 10.1016/S0254-0584(99)00169-8 [8] ZHANG C L, LI S, WANG L J, WU T H, PENG S Y. Studies on the decomposition of carbon dioxide into carbon with oxygen-deficient magnetite Ⅱ. The effects of properties of magnetite on activity of decomposition CO2 and mechanism of the reaction[J]. Mater Chem Phys, 2000, 62(1):52-61. doi: 10.1016/S0254-0584(99)00168-6 [9] ZHU L, YAO K L, LIU Z L. First-principles study of the polar(111) surface of Fe3O4[J]. Phys Rev B, 2006, 74(3):035409. doi: 10.1103/PhysRevB.74.035409 [10] LI Y L, YAO K L, LIU Z L. Structure, stability and magnetic properties of the Fe3O4(110) surface:Density functional theory study[J]. Surf Sci, 2007, 601(3):876-882. doi: 10.1016/j.susc.2006.10.037 [11] PENTCHCHEVA R, WENDLER F, MEYERHEIM H L, MORITZ W, JEDRECY N, SCHEFFLER M. Jahn-Teller stabilization of a "Polar" metal oxide surface:Fe3O4(001)[J]. Phys Rev Lett, 2005, 94(12):126101. doi: 10.1103/PhysRevLett.94.126101 [12] HUANG D M, CAO D B, LI Y W, JIAO H J. Density function theory study of CO adsorption on Fe3O4(111) surface[J]. J Phys Chem B, 2006, 110(28):13920-13925. doi: 10.1021/jp0568273 [13] YANG T, WEN X D, HUO C F, LI Y W, WANG J G, JIAO H J. Carburization of the Fe3O4(111) Surface[J]. J Phys Chem C, 2007, 112(16):6372-6379. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ021080213 [14] PAYNE M C, ALLAN D C, ARIAS T A, JOANNOPOULOS J D. Iterative minimization techniques for ab initio total-energy calculations:Molecular dynamics and conjugate gradients[J]. Rev Mod Phys, 1992, 64(4):1045-1097. doi: 10.1103/RevModPhys.64.1045 [15] MILMAN V, WINKLER B, WHITE J A, PICKARD C J, PAYNE M C, AKHMATASKAYA E V, NOBES R H. Electronic structure, properties, and phase stability of inorganic crystals:A pseudopotential plane-wave study[J]. Int J Quantum Chem, 2000, 77(5):895-910. doi: 10.1002/(ISSN)1097-461X [16] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1996, 77(18):3865-3868. doi: 10.1103/PhysRevLett.77.3865 [17] DUDAREV S L, BOTTON G A, SAVRASOV S Y, HUMPHREYS C J, SUTTON A P. Electron-energy-loss spectra and the structural stability of nickel oxide:An LSDA+U study[J]. Phys Rev B, 1998, 57(3):1505-1509. doi: 10.1103/PhysRevB.57.1505 [18] MENG Y, LIU X W, HUO C F, GUO W P, CAO D B, PENG Q, ALBERT D, XAVIER G, YANG Y, WANG J G, JIAO H J, LI Y W, WEN X D. When density functional approximations meet iron oxides[J]. J Chem Theory Comput, 2016, 12(10):5132-5144. doi: 10.1021/acs.jctc.6b00640 [19] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism[J]. Phys Rev B, 1990, 41(11):7892-7895. doi: 10.1103/PhysRevB.41.7892 [20] MONKHORST H J, PACK J D. Special points for Brillonin-zone integrations[J]. Phys Rev B, 1976, 13(12):5188-5192. doi: 10.1103/PhysRevB.13.5188 [21] LOUIE S G, FROYEN S, COHEN M L. Nonlinear ionic pseudopotentials in spin-density-functional calculations[J]. Phys Rev B, 1982, 26(4):1738-1742. doi: 10.1103/PhysRevB.26.1738 [22] NAYAK S K, NOOIJEN M, BERNASEK S L. Electronic structure study of CO adsorption on the Fe(001) surface[J]. J Phys Chem B, 2001, 105(1):164-172. doi: 10.1021/jp002314e [23] CHENG H S, REISER D B, DEAN S W JR, BAUMERT K. Structure and energetics of iron pentacarbonyl formation at an Fe(100) surface[J]. J Phys Chem B, 2001, 105(50):12547-12552. doi: 10.1021/jp0155112 [24] GE Q, JENKINS S J, KING D A. Localisation of adsorbate-induced demagnetisation:CO chemisorbed on Ni{110}[J]. Chem Phys Lett, 2000, 327(3/4):125-130. https://www.researchgate.net/publication/223123164_Localisation_of_adsorbate-induced_demagnetisation_CO_chemisorbed_on_Ni110 [25] WYCKOFF R W. Crystal Structures (Vol. 2)[M]. 2nd edition, 1982, p5. [26] SPENCER N D, SCHOONMAKER R C, SOMORJAI G A. Iron single crystals as ammonia synthesis catalysts:Effect of surface structure on catalyst activity[J]. J Catal, 1982, 74(1):129-135. doi: 10.1016/0021-9517(82)90016-1 [27] TOPSOE H, DUMESIC J A, BOUDART M. Alumina as a textural promoter of iron synthetic ammonia catalysts[J]. J Catal, 1973, 28(3):477-488. doi: 10.1016/0021-9517(73)90141-3 [28] LEMIRE C, MEYER R, HENRICH V E, SHAIKHUTDINOV S K, FREUND H J. The surface structure of Fe3O4(111) films as studied by CO adsorption[J]. Surf Sci, 2004, 572(1):103-114. doi: 10.1016/j.susc.2004.08.033 [29] CONDON N G, MURRAY P W, LEIBSLE F M, THORNTON G, LENNIE A R, VAUGHAN D J. Fe3O4(111) termination of α-Fe2O3(0001)[J]. Surf Sci, 1994, 310(1/3):L609-L613. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0233621150 [30] WEISS W, RANKE W. Surface chemistry and catalysis on well-defined epitaxial iron-oxide layers[J]. Prog Surf Sci, 2002, 70(1):1-151. http://cn.bing.com/academic/profile?id=1d3e7333c05bbca4e5a82f83e6a3b96e&encoded=0&v=paper_preview&mkt=zh-cn [31] SHAIKHUTDINOV S K, RITTER M, WANG X G, OVER H, WEISS W. Defect structures on epitaxial Fe3O4(111) films[J]. Phys Rev B, 1999, 60(15/16):11062-11069. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ029000372 [32] RITTER M, WEISS W. Fe3O4(111) surface structure determined by LEED crystallography[J]. Surf Sci, 1999, 432(1/2):81-94. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ025546280 [33] WANG S G, CAO D B, LI Y W, WANG J G, JIAO H J. Chemisorption of CO2 on nickel surfaces[J]. J Phys Chem B, 2005, 109(40):18956-18963. doi: 10.1021/jp052355g [34] FREUND H J, MESSMER R P. On the bonding and reactivity of CO2 on metal surfaces[J]. Surf Sci, 1986, 172(1):1-30. doi: 10.1016/0039-6028(86)90580-7 [35] GOPEL W, ROCKER G. Localized and delocalized charge transfer during adsorption on semiconductors:Experiments and cluster calculations on the prototype surface ZnO(1010)[J]. J Vac Sci Technol, 1982, 21(2):389-397. doi: 10.1116/1.571788 [36] GOPEL W. Chemisorption and charge transfer at ionic semiconductor surfaces:Implication in desiging gas sensors[J]. Prog Surf Sci, 1985, 20(1):9-103. doi: 10.1016/0079-6816(85)90004-8 [37] RUNGE F, GOPEL W. Comparative study on the reactivity of polycrystalline and single crystal ZnO surfaces:O2 and CO2 interaction[J]. Z Phys Chem, 1980, 123(2):173-192. doi: 10.1524/zpch.1980.123.2.173 [38] HOTAN W, GOPEL W, HAUL R. Interaction of CO2 and CO with nonpolar Zinc oxide surfaces[J]. Surf Sci, 1979, 83(1):162-180. doi: 10.1016/0039-6028(79)90486-2 -





 下载:
下载: