Effect of Cu promoter on polyvinyl alcohol-assisted preparation of iron catalyst for Fischer-Tropsch synthesis
-
摘要: 通过共沉淀法或聚乙烯醇(PVA)辅助共沉淀法分别制备了Fe2O3和FeCu催化剂,结合BET、XRD、SEM、H2-TPR等表征手段,研究了Cu助剂对PVA辅助的沉淀铁催化剂的织构性质、物相结构、形貌特征、还原行为以及F-T合成反应性能的影响。结果表明,Cu助剂的加入增大了铁基催化剂中α-Fe2O3的晶粒,减小了催化剂的BET比表面积和孔容,增大了孔径;改变了铁基催化剂的形貌;促进了铁基催化剂在H2中的还原。反应过程中,在催化剂中只添加Cu助剂时,有利于提高催化剂的反应活性,而当同时加入Cu助剂和PVA时,由于Cu助剂与PVA较强的相互作用,反而降低了催化剂的反应活性,且催化剂的选择性向轻质烃方向偏移。Abstract: Fe2O3 or FeCu catalysts were prepared by co-precipitation method with or without the assistance of polyvinyl alcohol (PVA). The effect of Cu promoter on the structure and catalytic behaviour of the catalysts were investigated. The catalysts were characterized by BET, SEM, XRD, H2-TPR and FT-IR techniques. The results showed that the addition of Cu promoter could enhance the crystal growth of α-Fe2O3, decrease BET surface area and pore volume and increase pore size and promote the reduction of the catalysts in H2. In addition, Cu promoter remarkably influenced the surface morphology. In Fischer-Tropsch synthesis (FTS) reaction, the catalytic activity was increased when Cu promoter was added only. The addition of Cu promoter and PVA decreased the FTS activity, and shifted the hydrocarbon products to lighter molecular weight.
-
Key words:
- Cu promoter /
- PVA /
- co-precipitation method /
- Fischer-Tropsch synthesis
-
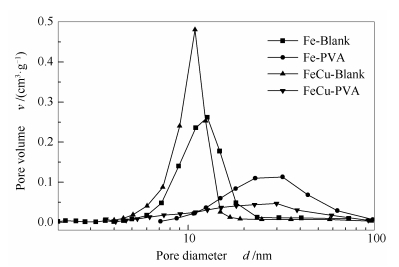
表 1 氧化态催化剂的织构性质
Table 1 Textural properties of the catalysts as-prepared
Catalyst BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average pore size d/nm Fe-Blank 19.75 0.08 13.75 Fe-PVA 11.46 0.07 24.06 FeCu-Blank 24.02 0.09 14.68 FeCu-PVA 7.78 0.04 19.36 -
[1] YANG J, MA W, CHEN D, HOLMEN A, DAVIS B H. Fischer-Tropsch synthesis:A review of the effect of CO conversion on methane selectivity[J]. Appl Catal A:Gen, 2014, 470(0):250-260. http://www.sciencedirect.com/science/article/pii/S0926860X13006807 [2] WANG T, WANG S G, LUO Q Q, LI Y W, WANG J G, BELLER M, JIAO H J. Hydrogen adsorption structures and energetics on iron surfaces at high coverage[J]. J Phys Chem C, 2014, 118(8):4181-4188. doi: 10.1021/jp410635z [3] SUO H Y, WANG S G, ZHANG C H, XU J, WU B S, YANG Y, XIANG H W, LI Y W. Chemical and structural effects of silica in iron-based Fischer-Tropsch synthesis catalysts[J]. J Catal, 2012, 286(0):111-123. http://www.sciencedirect.com/science/article/pii/S0021951711003599 [4] DRY M E. Present and future applications of the Fischer-Tropsch process[J]. Appl Catal A:Gen, 2004, 276(1/2):1-3. http://www.sciencedirect.com/science/article/pii/S0926860X04007276 [5] DRY M E, HOOGENDOORN J. Technology of the Fischer-Tropsch process[J]. Cat Rev-Sci Eng, 1981, 23(1/2):265-278. doi: 10.1080/03602458108068078 [6] LI S, LI A, KRISHNAMOORTHY S, IGLESIA E. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts[J]. Catal Lett, 2001, 77(4):197-205. doi: 10.1023/A:1013284217689 [7] de SMIT E, de GROOT F M, BLUME R, HÄVECKER M, KNOP-GERICKE A, WECKHUYSEN B M. The role of Cu on the reduction behavior and surface properties of Fe-based Fischer-Tropsch catalysts[J]. PCCP, 2010, 12(3):667-680. doi: 10.1039/B920256K [8] WIELERS A, KOEBRUGGE G, GEUS J. On the properties of silica-supported bimetallic Fe-Cu catalysts Part Ⅱ. Reactivity in the Fischer-Tropsch synthesis[J]. J Catal, 1990, 121(2):375-385. doi: 10.1016/0021-9517(90)90246-G [9] WIELERS A, HOP C, VAN BEIJNUM J, VAN der KRAAN A, GEUS J. On the properties of silica-supported bimetallic Fe-Cu catalysts Part Ⅰ. Preparation and characterization[J]. J Catal, 1990, 121(2):364-374. doi: 10.1016/0021-9517(90)90245-F [10] WAN H J, WU B S, ZHANG C H, XIANG H W, LI Y W. Promotional effects of Cu and K on precipitated iron-based catalysts for Fischer-Tropsch synthesis[J]. J Mol Catal A:Chem, 2008, 283(1/2):33-42. http://www.sciencedirect.com/science/article/pii/S1381116907006930 [11] MA C L, DONG G H, CHEN J G. Effect of PVA concentration on structure and performance of precipitated iron-based catalyst for Fischer-Tropsch synthesis[J]. J Braz Chem Soc, 2017, 28(8):1564-1572. https://www.researchgate.net/publication/313113304_Effect_of_PVA_Concentration_on_Structure_and_Performance_of_Precipitated_Iron-Based_Catalyst_for_Fischer-Tropsch_Synthesis [12] 马彩莲. 新型铁基催化剂的制备化学及F-T合成反应性能研究[D]. 山西: 中国科学院山西煤炭化学研究所, 2015. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y2956502MA Cai-lian. Preparation chemistry and Fischer-Tropsch synthesis performance of a novel iron catalyst[D]. Shanxi: Institute of Coal Chemistry, Chinese Academy of Sciences, 2015. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y2956502 [13] SUO H Y, ZHANG C H, WU B S, XU J, YANG Y, XIANG H W, LI Y W. A comparative study of Fe/SiO2 Fischer-Tropsch synthesis catalysts using tetraethoxysilane and acidic silica sol as silica sources[J]. Catal Today, 2012, 183(1):88-95. doi: 10.1016/j.cattod.2011.08.047 [14] YANG Y, XIANG H W, XU Y Y, BAI L, LI Y W. Effect of potassium promoter on precipitated iron-manganese catalyst for Fischer-Tropsch synthesis[J]. Appl Catal A:Gen, 2004, 266(2):181-194. doi: 10.1016/j.apcata.2004.02.018 [15] QIN S D, ZHANG C H, XU J, WU B S, XIANG H W, LI Y W. Effect of Mo addition on precipitated Fe catalysts for Fischer-Tropsch synthesis[J]. J Mol Catal A:Chem, 2009, 304(1/2):128-134. http://www.sciencedirect.com/science/article/pii/S1381116909000612 [16] MOGOROSI R P, FISCHER N, CLAEYS M, VAN STEEN E. Strong-metal-support interaction by molecular design:Fe-silicate interactions in Fischer-Tropsch catalysts[J]. J Catal, 2012, 289:140-150. doi: 10.1016/j.jcat.2012.02.002 [17] COZAR O, LEOPOLD N, JELIC C, CHIS V, DAVID L, MOCANU A, TOMOAIA-COTISEL M. IR, Raman and surface-enhanced Raman study of desferrioxamine B and its Fe (Ⅲ) complex, ferrioxamine B[J]. J Mol Struct, 2006, 788(1):1-6. http://www.sciencedirect.com/science/article/pii/S0022286005003777 [18] LI S, ZHU H Q, QIN Z F, WANG G F, ZHANG Y G, WU Z W, LI Z K, CHEN G, DONG W W, WU Z H. Morphologic effects of nano CeO2-TiO2 on the performance of Au/CeO2-TiO2 catalysts in low-temperature CO oxidation[J]. Appl Catal B:Environ, 2014, 144:498-506. doi: 10.1016/j.apcatb.2013.07.049 -





 下载:
下载: