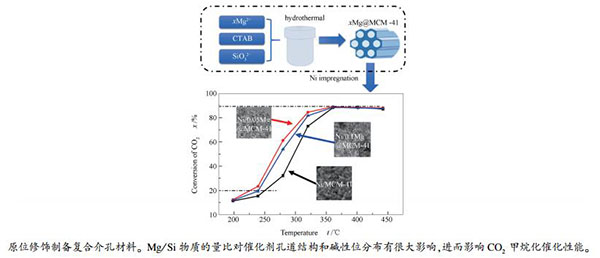
-
摘要: 通过原位引入Mg一步法合成了Mg@MCM-41复合介孔材料,并将其作为载体制备了高性能Ni基CO2甲烷化催化剂。通过BET、XRD、TEM、CO2-TPD、TG等手段对催化剂进行了表征分析,着重比较了Mg/Si物质的量比对于催化剂特性的影响。结果表明,当Mg/Si物质的量比为0.05时能够在不破坏孔道结构的前提下显著增加催化剂上的碱性位,有效地提高了催化剂对CO2的吸附和活化,从而促进CO2甲烷化反应过程中反应物的转化。实验所制得的催化剂均具有较好的热稳定性和催化反应活性,其中,Ni/0.05Mg@MCM-41在CO2甲烷化反应表现出最优的催化性能,在320 ℃,1 MPa的条件下,CO2转化率和CH4选择性分别高达84.3%和97.8%。Abstract: A series of xMg@MCM-41(x=0, 0.05, 0.1) functional mesoporous materials were synthesized by a novel in-situ one pot method and then were used as support for Ni based catalysts. The results of XRD and TEM show that when the amount of Mg/Si (molar ratio) is 0.05, Mg@MCM-4 with a regular and ordered mesoporous structure is synthesized where Mg is introduced into the framework of MCM-41. Introducing Mg into the framework of the support can significantly enhance the basic properties of the catalyst, thus promoting the adsorption and activation of CO2. The catalysts prepared in the experiments all have good thermal stability and catalytic activity. Among them, Ni/0.05Mg@MCM-41 shows the best low temperature reaction activity in the CO2 methanation reaction.
-
Key words:
- CO2 /
- methanation /
- in-situ /
- Mg@MCM-41 /
- catalytic activity
-
表 1 催化剂的物理化学特性
Table 1 Physicochemical properties of the catalysts
Catalyst ABET
/(m2·g-1)dpore/
nmvpore/
(cm3·g-1)Mg/Si
(molar ratio)Theoretical/% Actual w/% dNia/nm Mg Ni Mg Ni Ni/MCM-41 622.5 2.31 0.95 0 0 8.7 0.00 8.18 20 Ni/0.05Mg@MCM-41 606.3 3.37 0.84 0.05 1.78 8.7 1.94 8.28 15 Ni/0.1Mg@MCM-41 498.5 3.12 0.81 0.1 3.48 8.7 3.41 8.45 15 a: Ni size calculated by XRD -
[1] SONG Q, ZHOU Z, HE L. Efficient, selective and sustainable catalysis of carbon dioxide[J]. Green Chem, 2017, 19(16):3707-3728. doi: 10.1039/C7GC00199A [2] FRONTERA P, MACARIO A, FERRARO M, ANTONUCCI P. Supported catalysts for CO2 methanation:A review[J]. Catalysts, 2017, 7:(2). [3] BAILERA M, LISBONA P, ROMEO L M, ESPATOLERO S. Power to gas projects review:Lab, pilot and demo plants for storing renewable energy and CO2[J]. Renewable Sustainable Energy Rev, 2017, 69:292-312. doi: 10.1016/j.rser.2016.11.130 [4] CHEN J, WANG M, WANG S, LI X. Hydrogen production via steam reforming of acetic acid over biochar-supported nickel catalysts[J]. Int J Hydrogen Energy, 2018, 4(39):18160-18168. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c051cd10f229ef4e87bc3f8ad7427c48 [5] AZIZ M A A, JALIL A A, TRIWAHYONO S, SAAD M W A. CO2 methanation over Ni-promoted mesostructured silica nanoparticles:Influence of Ni loading and water vapor on activity and response surface methodology studies[J]. Chem Eng J, 2015, 260:757-764. doi: 10.1016/j.cej.2014.09.031 [6] ZHU L, YIN S, YIN Q, WANG H, WANG S. Biochar:A new promising catalyst support using methanation as a probe reaction[J]. Energy Sci Eng, 2015, 3(2):126-134. http://d.old.wanfangdata.com.cn/Periodical/zghjkx201610032 [7] LI W, WANG H, JIANG X, ZHU J, LIU Z, GUO X, SONG C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts[J]. Rsc Adv, 2018, 8(14):7651-7669. doi: 10.1039/C7RA13546G [8] YIN S, ZHU L, LIU Y, WANG S. The effect of titanium source on methanation over Ni/TiO2 catalysts[J]. Chem Res Chin Univ, 2017, 34:(2). [9] LEE G D, MOON M J, PARK J H, PARK S S, HONG S S. Raney Ni catalysts derived from different alloy precursors Part II. CO and CO2 methanation activity[J]. Korean J Chem Eng, 2005, 22(4):541-546. [10] BAYSAL Z, KURETI S. CO2 methanation on Mg-promoted Fe catalysts[J]. Appl Catal B:Environ, 2020, 262:118300. doi: 10.1016/j.apcatb.2019.118300 [11] ALI S H, GIURCO D, ARNDT N, NICKLESS E, BROWN G, DEMETRIADES A, DURRHEIM R, ENRIQUEZ M A, KINNAIRD J, LITTLEBOY A, MEINERT L D, OBERHÄNSLI R, SALEM J, SCHODDE R, SCHNEIDER G, VIDAL O, YAKOVLEVA N. Mineral supply for sustainable development requires resource governance[J]. Nature, 2017, 543(7645):367-372. doi: 10.1038/nature21359 [12] AZIZ M A A, JALIL A A, TRIWAHYONO S, AHMAD A. CO2 methanation over heterogeneous catalysts:Recent progress and future prospects[J]. Green Chem, 2015, 17(5):2647-2663. doi: 10.1039/C5GC00119F [13] WANG W, GONG J. Methanation of carbon dioxide:An overview[J]. Front Chem Sci Eng, 2011, 5(1):2-10. doi: 10.1007/s11705-010-0528-3 [14] ZHAO D, SUN J, LI Q, STUCKY G D. Morphological control of highly ordered mesoporous silica SBA-15[J]. Chem Mater, 2000, 12(2):275-279. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=86ee40dbb9a39bdbef64f3094ed53900 [15] BECK J S, VARTULI J C, ROTH W J, LEONOWICZ M E, KRESGE C T, SCHMITT K D, CHU C T W, OLSON D H, SHEPPARD E W. A new family of mesoporous molecular sieves prepared with liquid crystal templates[J]. J Am Chem Soc, 1992, 114(27):10834-10843. doi: 10.1021/ja00053a020 [16] BACARIZA M C, GRACA I, BEBIANO S S, LOPES J M, HENRIQUES C. Micro- and mesoporous supports for CO2 methanation catalysts:A comparison between SBA-15, MCM-41 and USY zeolite[J]. Chem Eng Sci, 2018, 175:72-83. doi: 10.1016/j.ces.2017.09.027 [17] 张加赢, 辛忠, 孟鑫, 陶淼.基于MCM-41的镍基甲烷化催化剂活性与稳定性[J].化工学报, 2014, 65(1):160-168. doi: 10.3969/j.issn.0438-1157.2014.01.020ZHANG Jia-ying, XIN Zhong, MENG Xin, TAO Miao. Activity and stability of nickel based MCM-41 methanation catalysts for production of synthetic natural gas[J]. CIESC J, 2014, 65(1):160-168. doi: 10.3969/j.issn.0438-1157.2014.01.020 [18] TAN J, WANG J, ZHANG Z, MA Z, WANG L, LIU Y. Highly dispersed and stable Ni nanoparticles confined by MgO on ZrO2 for CO2 methanation[J]. Appl Surf Sci, 2019, 481:1538-1548. doi: 10.1016/j.apsusc.2019.03.217 [19] 曾艳, 马宏方, 张海涛, 应卫勇, 房鼎业.燃烧法制备Ni基甲烷化催化剂:Mg、Mn和La助剂对催化性能的影响[J].天然气化工(C1化学与化工), 2015, 40(4):6-10. doi: 10.3969/j.issn.1001-9219.2015.04.002ZENG Yan, MA Hong-fang, ZHANG Hai-tao, YING Wei-yong, FANG Ding-ye. Ni-based methanation catalysts prepared by solution combustion method:Effect of Mg, Mn and La promoters[J]. Nat Gas Ind, 2015, 40(4):6-10. doi: 10.3969/j.issn.1001-9219.2015.04.002 [20] BACARIZA M C, GRACA I, BEBIANO S S, LOPES J M, HENRIQUES C. Magnesium as promoter of CO2 methanation on Ni-Based USY zeolites[J]. Energy Fuels, 2017, 31(9):9776-9789. doi: 10.1021/acs.energyfuels.7b01553 [21] XU L, WANG F, CHEN M, YANG H, NIE D, QI L, LIAN X. Alkaline-promoted Ni based ordered mesoporous catalysts with enhanced low-temperature catalytic activity toward CO2 methanation[J]. Rsc Adv, 2017, 7(30):18199-18210. doi: 10.1039/C7RA01673E [22] WANG X, ZHU L, ZHUO Y, ZHU Y, WANG S. Enhancement of CO2 methanation over La-Modified Ni/SBA-15 catalysts prepared by different doping methods[J]. ACS Sustainable Chem Eng, 2019, 7:14647-14660. doi: 10.1021/acssuschemeng.9b02563 [23] WANG X, ZHU L, LIU Y, WANG S. CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2[J]. Sci Total Environ, 2018, 625:686-695. doi: 10.1016/j.scitotenv.2017.12.308 [24] LIU Y, ZHU L, WANG S, FUKUDA S. Bio-MCM-41:A high-performance catalyst support derived from pyrolytic biochar[J]. New J Chem, 2018, 42(15):12394-12402. doi: 10.1039/C8NJ01063C [25] WANG X, LIU Y, ZHU L, LI Y, WANG K, QIU K, TIPPAYAWONG N, AGGARANGSI P, REUBROYCHAROEN P, WANG S. Biomass derived N-doped biochar as efficient catalyst supports for CO2 methanation[J]. J CO2 Util, 2019, 34:733-741. doi: 10.1016/j.jcou.2019.09.003 [26] 洪新, 唐克.杂原子介孔Ce-MCM-41分子筛的制备及其吸附脱除甲硫醚性能[J].燃料化学学报, 2015, 43(4):456-461. doi: 10.3969/j.issn.0253-2409.2015.04.013HONG Xin, TANG Ke. Preparation of heteroatomic mesoporous Ce-MCM-41 molecular sieve and its performance in the adsorptive removal of dimethyl sulfide[J]. J Fuel Chem Technol, 2015, 43(4):456-461. doi: 10.3969/j.issn.0253-2409.2015.04.013 [27] JIA W, LIU T, LI Q, YANG J. Highly efficient photocatalytic reduction of CO2 on surface-modified Ti-MCM-41 zeolite[J]. Catal Today, 2019, 335:221-227. doi: 10.1016/j.cattod.2018.11.046 [28] MARLER B, OBERHAGEMANN U, VORTMANN S, GIES H. Influence of the sorbate type on the XRD peak intensities of loaded MCM-41[J]. Microporous Mater, 1996, 6(5/6):375-383. doi: 10.1016-0927-6513(96)00016-8/ [29] SONG X, GUAN Q, SHU Y, ZHANG X, LI W. Facile in-situ encapsulation of highly dispersed Ni@MCM-41 for the trans-decalin production from hydrogenation of naphthalene at low temperature[J]. ChemCatChem, 2019, 11(4):1286-1294. doi: 10.1002/cctc.201801788 [30] HAMMOND W, PROUZET E, MAHANTI S D, PINNAVAIA T J. Structure factor for the periodic walls of mesoporous MCM-41 molecular sieves[J]. Microporous Mesoporous Mater, 1999, 27(1):19-25. doi: 10.1016/S1387-1811(98)00222-4 [31] ZHANG M, LIU Z, LIN G, ZHANG H. Pd/CNT-promoted CuZrO2/HZSM-5 hybrid catalysts for direct synthesis of DME from CO2/H2[J]. Appl Catal A:Gen, 2013, 451:28-35. doi: 10.1016/j.apcata.2012.10.038 [32] 张旭, 孙文晶, 储伟.等离子体技术对CO2甲烷化用Ni/SiO2催化剂的改性作用[J].燃料化学学报, 2013, 41(1):96-101. doi: 10.3969/j.issn.0253-2409.2013.01.016ZHANG Xu, SUN Wen-jing, CHU Wei. Effect of glow discharge plasma treatment on the performance of Ni/SiO2 catalyst in CO2 methanation[J]. J Fuel Chem Technol, 2013, 41(1):96-101. doi: 10.3969/j.issn.0253-2409.2013.01.016 [33] SONG J, SUN Y, BA R, HUANG S, ZHAO Y, ZHANG J, SUN Y, ZHU Y. Monodisperse Sr-La2O3 hybrid nanofibers for oxidative coupling of methane to synthesize C2 hydrocarbons[J]. Nanoscale, 2015, 7(6):2260-2264. doi: 10.1039/C4NR06660J [34] OCAMPO F, LOUIS B, KIWI-MINSKER L, ROGER A-C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1-xO2 catalysts for carbon dioxide methanation[J]. Appl Catal A:Gen, 2011, 392(1):36-44. [35] 朱秋军, 王海洋, 李振花. Ni基催化剂上CO甲烷化反应性能研究[J].天然气化工(C1化学与化工), 2012, 37(2):21-24. doi: 10.3969/j.issn.1001-9219.2012.02.004ZHU Qiu-jun, WANG Hai-yang, LI Zhen-hua. Study on methanation of carbon monoxide over nickel-based catalysts[J]. Nat Gas Ind, 2012, 37(2):17-20. doi: 10.3969/j.issn.1001-9219.2012.02.004 -





 下载:
下载: