Preparation of nanometer CuO-ZnO-ZrO2 catalysts through citrate-gel process and their catalytic properties for methanol synthesis from CO2
-
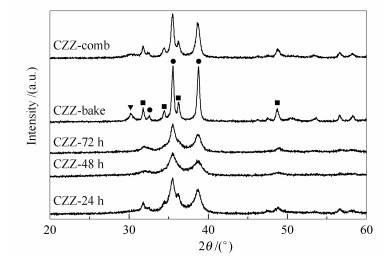
摘要: 采用柠檬酸盐凝胶法制备出纳米CuO-ZnO-ZrO2(CZZ) 催化剂, 应用XPS、BET、XRD、H2-TPR、H2-TPD、CO2-TPD和TG-DTA等检测手段对催化剂及前驱体的结构进行表征.研究了湿凝胶干燥时间和柠檬酸用量对催化剂结构的影响, 并与燃烧法制得的催化剂进行对比, 考察了不同催化剂CO2加氢制甲醇的性能.研究表明, 延长湿凝胶干燥时间可有效防止催化剂焙烧时发生喷溅, 有利于催化剂中各组分的分散, 提高催化剂对H2和CO2的吸附能力; 112℃干燥48h制得的催化剂(CZZ-48h) BET比表面积为43.5m2/g, 高于燃烧法; 柠檬酸用量等于化学计量比时催化剂的性能最佳, 在240℃、2.6MPa、空速为3600h-1、H2/CO2(体积比) 为3的条件下甲醇时空收率达109.4g/(kg·h); 柠檬酸过量会影响催化剂组分的分散度, 并造成分解残留覆盖催化剂表面活性位而不利于CO2加氢反应.
-
关键词:
- 柠檬酸盐凝胶法 /
- CuO-ZnO-ZrO2 /
- CO2催化加氢 /
- 甲醇
Abstract: CuO-ZnO-ZrO2(CZZ) nanocatalysts were successfully prepared by citrate-gel method. The catalysts and their precursors were characterized by X-ray photoelectron spectroscopy (XPS), N2 adsorption specific surface area measurement (BET), X-ray diffraction (XRD), H2-temperature-programmed reduction (H2-TPR), H2 and CO2-temperature-programmed desorption (H2 and CO2-TPD) and thermogravimetric analysis (TG-DTA). Drying time of the wet gel and the dosage of citric acid were systematicly studied, while combustion method was also conducted with the comparison of those obtained catalysts. Results show that, prolonged drying process can effectively prevent particle spattering during calcination, benefit the dispersion of different components in the catalyst, and improve the adsorption ability of catalyst for H2 and CO2. Sample CZZ-48h, which was dried at 112℃, 48h, maintained a much higher BET specific surface area than that prepared by combustion method. The CuO-ZnO-ZrO2 catalyst, in which 100% of stoichiometric amount of citric acid was added, exhibited an optimum catalytic behavior with a space-time-yield of methanol 109.4g·h-1·kg-1 under the condition of 240℃, 2.6 MPa, 3600h-1, H2/CO2=3. The detriment of the catalytic performance excessive amounts of citric acid is ascribed to decline dispersion of the catalyst component, and decomposition residual covering on the surface active species of the catalyst.-
Key words:
- citrate-gel process /
- CuO-ZnO-ZrO2 /
- CO2 catalytic hydrogenation /
- methanol
-
表 1 不同Cu-ZnO-ZrO2催化剂的催化性能
Table 1 Catalytic properties of different Cu-ZnO-ZrO2 catalysts
Catalyst CO2
conversation
x/%CH3OH
selectivity
s/%WTY of CH3OH
/(g· kg-1· h-1)CZZ-24 h 22.2 27.4 78.3 CZZ-48 h 22.3 38.1 109.4 CZZ-72 h 24.5 33.5 105.6 CZZ-bake 19.9 23.9 44.4 CZZ-comb 12.9 26.8 61.3 CZZ-125 26.4 24.5 83.2 CZZ-150 27.0 23.6 81.9 reaction conditions: t=240 ℃, p=2.6 MPa, WHSV=3 600 h-1, and CO2/H2(mol ratio)=1:3 表 2 不同凝胶干燥时间和方式制备CZZ催化剂的物化性质
Table 2 Physicochemical properties of calcined CZZ catalysts
Catalyst ABET
/(m2·g-1)v
/(cm3·g-1)CuO crystallite size
d /nmCZZ-24 h 38.7 0.20 14.9 CZZ-48 h 43.5 0.17 11.8 CZZ-72 h 39.7 0.09 12.0 CZZ-bake 15.9 0.09 23.9 CZZ-comb 16.3 0.06 16.2 表 3 催化剂的还原峰温度及还原峰在H2-TPR谱图中所占的面积比例
Table 3 Temperatures of reduction peaks and their contributions to the H2-TPR profiles over catalysts
Catalyst tα /℃ tβ /℃ Aα/(Aα+Aβ) /% CZZ-24 h 238 257 72 CZZ-48 h 235 257 75 CZZ-72 h 232 257 73 CZZ-bake 226 257 59 CZZ-comb 230 257 74 Aα and Aβ represent the areas of α and β peaks,respectively 表 4 不同柠檬酸用量制备CuO-ZnO-ZrO2的XPS数据
Table 4 Surface concentrations of CZZ catalysts prepared from different amounts of citric acid
Catalyst Surface concentrations wat/% Cu 2p Zn 2p Zr 3d C 1s O 1s CZZ-100 19.6 11.9 6.0 0 62.6 CZZ-125 17.1 10.3 4.1 15.2 53.3 CZZ-150 16.2 9.0 4.1 13.3 57.5 -
[1] OLAH G A, PRAKASH G K S, GOEPPERT A. Anthropogenic chemical carbon cycle for a sustainable future[J]. J Am Chem Soc, 2011, 133(33): 12881-12898. doi: 10.1021/ja202642y [2] OLAH G A. Towards oil independence through renewable methanol chemistry[J]. AngewChemInt Ed, 2013, 52(1): 104-107. [3] MEITZNER G, IGLESIA E. New insights into methanol synthesis catalysts from X-ray absorption spectroscopy[J]. Catal Today, 1999, 53(3): 433-441. doi: 10.1016/S0920-5861(99)00135-2 [4] 丛昱, 包信和, 张涛, 孙孝英, 梁东白, 田金忠, 黄宁表.超细Cu-ZnO-ZrO2催化剂上甲醇合成的TPSR和TPD研究[J].燃料化学学报, 2000, 28(3): 238-243. http://www.cqvip.com/Main/Detail.aspx?id=4348396CONG Yu, BAO Xin-he, ZHANG Tao, SUN Xiao-ying, LIANG Dong-bai, TIAN Jin-zhong, HUANG Ning-biao. TPSR and TPD studies of ultrafine Cu-ZnO-ZrO2 catalysts for methanol synthesis[J]. J Fuel Chem Technol, 2000, 28(3): 238-243. http://www.cqvip.com/Main/Detail.aspx?id=4348396 [5] SUN Q, LIU C W, PAN W, ZHU Q M, DENG J F. In situ IR studies on the mechanism of methanol synthesis over an ultrafine Cu/ZnO/Al2O3 catalyst[J]. ApplCatal A: Gen, 1998, 171: 301-308. [6] 丛昱, 田金忠, 黄宁表, 徐长海, 张涛, 孙孝英, 关文, 梁东白.超细Cu-ZnO-ZrO2催化剂的制备及其催化CO2加氢合成甲醇的性能[J].催化学报, 2000, 21(3): 247-250. http://d.wanfangdata.com.cn/Periodical/cuihuaxb200003014CONG Yu, TIAN Jin-zhong, HUANG Ning-biao, XU Zhang-hai, ZHANG Tao, SUN Xiao-ying, GUAN Wen, LIANG Dong-bai. Preparation of ultrafine Cu-ZnO-ZrO2catalysts and CO2 hydrogenation performance[J]. Chin J Catal, 2000, 21(3): 247-250. http://d.wanfangdata.com.cn/Periodical/cuihuaxb200003014 [7] ARENA F, BARBERA K, ITALIANO G, BONURAG, SPADARO L, FRUSTERI F. Synthesis, characterization and activity pattern of Cu-ZnO/ZrO2 catalystsin the hydrogenation of carbon dioxide to methanol[J]. J Catal, 2007, 249(2): 185-194. doi: 10.1016/j.jcat.2007.04.003 [8] LI C M, YUAN X D, FUJIMOTO K. Development of highly stable catalyst for methanol synthesis from carbon dioxide[J]. Appl Catal A: Gen, 2014, 469: 306-311. doi: 10.1016/j.apcata.2013.10.010 [9] GAO P, LI F, XIAO F K, ZHAO N, WEI W, ZHONG L S, SUN Y H. Effect of hydrotalcite-containing precursors on the performance of Cu/Zn/Al/Zr catalysts for CO2 hydrogenation: Introduction of Cu2+ at different formation stages of precursors[J]. Catal Today, 2012, 194(1): 9-15. doi: 10.1016/j.cattod.2012.06.012 [10] YANG R Q, YU X C, ZHANG Y, LI W Z, TSUBAKI N. A new method of low-temperature methanol synthesis on Cu/ZnO/Al2O3 catalysts from CO/CO2/H2[J]. Fuel, 2008, 87(4/5): 443-450. [11] GUO X M, MAO D S, WANG S, WU G S, LU G Z. Combustion synthesis of CuO-ZnO-ZrO2 catalysts for the hydrogenation of carbon dioxide to methanol[J]. Catal Commun, 2009, 10(13): 1661-1664. doi: 10.1016/j.catcom.2009.05.004 [12] 郭晓明, 毛东森, 卢冠忠, 王嵩. CuO-ZnO-ZrO2的柠檬酸燃烧法制备及其催化CO2加氢合成甲醇的性能[J].物理化学学报, 2012, 28(1): 170-176. http://www.whxb.pku.edu.cn/CN/abstract/abstract27824.shtmlGUO Xiao-ming, MAO Dong-sen, LU Guan-zhong, WANG Song.Preparation of CuO-ZnO-ZrO2 by citric acid combustion method and its catalytic property for methanol synthesis from CO2 hydrogenation[J]. Acta Phys-Chem Sin, 2012, 28(1): 170-176. http://www.whxb.pku.edu.cn/CN/abstract/abstract27824.shtml [13] SHI L, YANG R Q, TAO K, YONEYAMA Y, TAN Y S, TSUBAKI N. Surface impregnation combustion method to prepare nanostructured metallic catalysts without further reduction: As-burnt Cu-ZnO/SiO2 catalyst for low-temperature methanol synthesis[J]. Catal Today, 2012, 185(1): 54-60. doi: 10.1016/j.cattod.2011.10.015 [14] 朱毅青, 文艺, 赖梨芳, 宗封琦, 王剑.超细CuO/ZnO/TiO2-SiO2的表征和CO2加氢合成甲醇性能研究[J].燃料化学学报, 2004, 32(4): 486-491. http://rlhxxb.sxicc.ac.cn/EN/Y2004/V32/I04/486ZHU Yi-qing, WEN Yi, LAI Li-fang, ZONG Feng-qi, WANG Jian. Characterization and catalytic activity evaluation of ultrafine CuO/ZnO/TiO2-SiO2 catalysts for CO2 hydrogenation to methanol[J]. J Fuel Chem Technol, 2004, 32(4): 486-491. http://rlhxxb.sxicc.ac.cn/EN/Y2004/V32/I04/486 [15] JUN K W, SHEN W J, RAO KS R, LEE K W. Residual sodium effect on the catalytic activity of Cu/ZnO/Al2O3 in methanol synthesis from CO2 hydrogenation[J]. Appl Catal A: Gen, 1998, 174: 231-238. doi: 10.1016/S0926-860X(98)00195-1 [16] 孔秀琴, 唐兴江, 许珊, 王晓来.溶胶-凝胶自燃烧法制备的CuO-ZnO/Al2O3及催化二氧化碳加氢制甲醇的性能研究[J].分子催化, 2013, 27(2): 159-165.KONG Xiu-qin, TANG Xing-jiang, XU Shan, WANG Xiao-lai. Preparation of CuO-ZnO/Al2O3 by sol-gel auto-combustion method and its catalytic property for methanol synthesis from CO2 hydrogenation[J]. J Mol Catal, 2013, 27(2): 159-165. [17] 林建新, 王自庆, 张留明, 倪军, 王榕, 魏可镁.柠檬酸络合法制备Ba促进ZrO2负载Ru催化剂上氨合成反应性能[J].催化学报, 2012, 33(7): 1075-1079. http://www.cqvip.com/QK/93027X/201207/42694980.htmlLIN Jian-xin, WANG Zi-qing, ZHANG Liu-ming, NI Jun, WANG Rong, WEI Ke-mei. Ammonia synthesis over ruthenium catalysts using barium-doped zirconia as supports prepared by citric acid method[J]. Chin J Catal, 2012, 33(7): 1075-1079. http://www.cqvip.com/QK/93027X/201207/42694980.html [18] 景茂祥, 沈湘黔, 沈裕军.柠檬酸盐凝胶法制备纳米氧化镍的研究[J].无机材料学报, 2004, 19(2): 289-294. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL200402004.htmJING Mao-xiang, SHEN Xiang-qian, SHEN Yu-jun.Preparation of nanometer nickel oxide by the citrate-gel process[J]. J Inorg Mater, 2004, 19(2): 289-294. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL200402004.htm [19] BONURA G, CORDARO M, CANNILLA C, ARENA F, FRUSTERI F. The changing nature of the active site of Cu-Zn-Zr catalysts for the CO2 hydrogenation reaction to methanol[J]. Appl Catal B: Environ, 2014, 152-153: 152-161. https://www.researchgate.net/publication/260166753_The_changing_nature_of_the_active_site_of_Cu-Zn-Zr_catalysts_for_the_CO2_hydrogenation_reaction_to_methanol [20] KARELOVIC A, BARGIBANT A, FERNÁNDEZ C, RUIZ P. Effect of the structural and morphological properties of Cu/ZnO catalysts prepared by citrate method on their activity toward methanol synthesis from CO2 and H2 under mild reaction conditions[J]. Catal Today, 2012, 197(1): 109-118. doi: 10.1016/j.cattod.2012.07.029 [21] 张鲁湘, 张永春, 陈绍云.助剂TiO2对CO2催化加氢制甲醇催化剂CuO-ZnO-Al2O3性能的影响[J].燃料化学学报, 2011, 39(12): 912-917. doi: 10.1016/S1872-5813(12)60002-4ZHANG Lu-xiang, ZHANG Yong-chun, CHEN Shao-yun. Effect of promoter TiO2 on the performance of CuO-ZnO-Al2O3 catalyst for CO2 catalytic hydrogenation to methanol[J]. J Fuel Chem Technol, 2011, 39(12): 912-917. doi: 10.1016/S1872-5813(12)60002-4 [22] ZHANG Y P, FEI J H, YU Y M, ZHENG X M. Methanol synthesis from CO2 hydrogenation over Cu based catalyst supported on zirconia modified Al2O3[J]. Energy Convers Manage, 2006, 47(18/19): 3360-3367. [23] DONG X, ZHANG H B, LIN G D, YUAN Y Z, TSAI K R. Highly active CNT-promoted Cu-ZnO-Al2O3 catalyst for methanol synthesis from H2/CO/CO2[J]. Catal Lett, 2003, 85(3): 237-246. [24] 李基涛, 张伟德, 陈明旦, 区泽棠.铜基催化剂上CO2吸附的TPD和TPSR研究[J].天然气化工, 1998, 23(5): 14-17.LI Ji-tao, ZHANG Wei-de, CHEN Ming-dan, QU Ze-tang. TPD and TPSR study of CO2 adsorption on Cu-based catalysts[J]. J Nat Gas Chem, 1998, 23(5): 14-17. [25] ARENA F, ITALIANO G, BARBERA K, BORDIGA S, BONURA G, SPADARO L, FRUSTERI F. Solid-state interactions, adsorption sites and functionality of Cu-ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH[J]. Appl Catal A: Gen, 2008, 350: 16-23. doi: 10.1016/j.apcata.2008.07.028 [26] 庄豪仁, 李承恩, 殷之文. PLZT柠檬酸盐前驱体的热分解[J].无机材料学报, 1988, 3(1): 27-31. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL198801004.htmZHUANG Hao-ren, LI Cheng-en, YIN Zhi-wen. Pyrolysis of PLZT citrate precursor[J]. J Inorg Mater, 1988, 3(1): 27-31. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL198801004.htm [27] SHI L, ZENG C Y, JIN Y Z, WANG T J, TSUBAKI N. A sol-gel auto-combustion method to prepare Cu/ZnO catalysts for low-temperature methanol synthesis[J]. Catal Sci Technol, 2012, 2: 2569-2577. doi: 10.1039/c2cy20423a [28] 徐征, 毛利群, 千载虎, 盛世善, 熊国兴. CO2加氢低压合成甲醇催化剂CuO/ZnO/ZrO2的光电子能谱研究[J].燃料化学学报, 1992, 20(3): 272-277. http://www.cnki.com.cn/Article/CJFDTotal-RLHX199203007.htmXU Zheng, MAO Li-qun, QIAN Zai-hu, SHENG Shi-shan, XIONG Guo-xing. Photoelectron spectroscopic study of CuO/ZnO/ZrO2 catalyst in low pressure methanol synthesis from CO2 and H2[J]. J Fuel Chem Technol, 1992, 20(3): 272-277. http://www.cnki.com.cn/Article/CJFDTotal-RLHX199203007.htm [29] BIESINGER M C, LAU L W M, GERSON A R, SMART R S C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn[J]. Appl Surf Sci, 2010, 257(3): 887-898. doi: 10.1016/j.apsusc.2010.07.086 -





 下载:
下载: