Experimental study on the mercury removal from flue gas using manganese modified titanium-zirconium and titanium-tin composite oxide catalysts
-
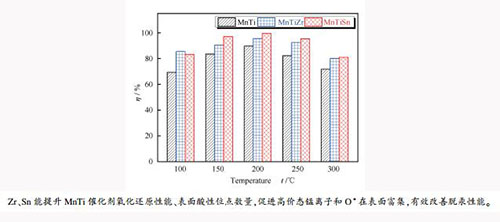
摘要: 采用共沉淀法制备了TiO2、TiZr和TiSn载体,浸渍锰制备了10% MnO2的MnTi、MnTiZr和MnTiSn催化剂。采用BET、XRD、H2-TPR、FT-IR和XPS等对样品进行表征,并对三种催化剂进行固定床脱汞性能实验。结果表明,在100-300℃,MnTiZr和MnTiSn催化剂脱汞性能均优于MnTi催化剂,这归因于Sn和Zr的引入提升了催化剂比表面积和低温氧化还原性能,增加了催化剂表面的酸性位点数量、高价态锰离子和O*含量;在反应温度为150-300℃,MnTiSn催化剂脱汞效率均高于MnTiZr催化剂,这是由于前者具有更好的氧化还原性能,表面具有更多含量的高价态锰离子、O*含量和酸性位点数量;在Hg0脱除过程中,催化剂表面活性组分如高价态锰离子和O*均消耗,参与了Hg0氧化为Hg2+的反应,且MnTiSn催化剂表面活性组分的消耗量更多。Abstract: In this study, TiO2, TiZr and TiSn supports were prepared using co-precipitation method, and MnTi, MnTiZr, and MnTiSn catalysts with MnO2 content of 10%were prepared by the wet impregnation method. BET, XRD, H2-TPR, FT-IR, and XPS were employed to characterize the prepared samples. The Hg0 removal performance tests over the three catalysts were conducted in a fixed-bed reactor apparatus. The results indicated that the Hg0 removal performance of MnTiZr and MnTiSn catalysts was better than that of MnTi catalyst in the temperature range of 100-300℃. This could be attributed to the introduction of Sn and Zr, which increased the specific surface area of the catalyst, improved the low-temperature redox performance of the catalyst, and elevate the number of acid sites, the high valence manganese ions concentration and O* content on the catalyst surface. The mercury removal efficiency of the MnTiSn catalyst was higher than that of MnTiZr catalyst at reaction temperature of 150-300℃, which could be ascribed to the higher redox performance of the MnTiSn catalyst and more content of the high valence manganese ions, O*, and surface acid sites on its surface. During the removal of Hg0 in flue gas by MnTiZr and MnTiSn catalysts, active ingredients on the catalyst surface such as high-valence manganese ions and O* were consumed and participated in the reaction of Hg0 oxidation to Hg2+. And the consumed amount of active ingredients on the surface of MnTiSn catalyst was more than that on the MnTiZr catalyst.
-
表 1 载体和催化剂的BET表征
Table 1 BET surface area, pore volume and pore size of the supports and the catalysts
Sample ABET/(m2·g-1) vt/(cm3·g-1) dave/nm TiO2 66.38 0.24 8.93 TiZr 213.64 0.38 6.97 TiSn 87.53 0.29 9.65 MnTi 41.46 0.21 9.32 MnTiZr 192.74 0.31 7.84 MnTiSn 73.16 0.25 10.43 表 2 催化剂表面原子摩尔含量
Table 2 Mole content of the atoms on the catalyst surface
Sample Mn 2p /% O 1s /% Mn2+/MnT Mn3+/MnT Mn4+/MnT OL/OT O*/OT Fresh MnTi 43.5 30.2 26.3 74.7 25.3 Fresh MnTiZr 39.1 27.3 33.6 60.4 39.6 Fresh MnTiSn 21.9 33.8 44.3 42.1 57.9 Spent MnTiZr 49.1 23.6 27.3 67.3 32.7 Spent MnTiSn 39.5 29.7 30.8 58.1 41.9 *: OT represents OL + O*, MnT represents Mn2+ + Mn3+ + Mn4+ -
[1] ZHAO J X, LI H L, YANG Z Q, ZHU L, ZHANG M G, FENG Y, QU W Q, YANG J P, SHIH K. Dual roles of nano-sulfide in efficient removal of elemental mercury from coal combustion flue gas within a wide temperature range[J]. Environ Sci Technol, 2018, 52(21):12926-12933. doi: 10.1021/acs.est.8b04340 [2] ZHAO S L, PUDASAINEE D, DUAN Y F, GUPTA R, LIU M, LU J H. A review on mercury in coal combustion process:Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies[J]. Prog Energy Combust Sci, 2019, 73:26-64. doi: 10.1016/j.pecs.2019.02.001 [3] 周强, 段钰锋, 卢平.燃煤电厂吸附剂喷射脱汞技术的研究进展[J].化工进展, 2018, 37(11):4460-4467. http://d.old.wanfangdata.com.cn/Periodical/hgjz201811044ZHOU Qiang, DUAN Yu-feng, LU Ping. Research progress on in-duct mercury removal by sorbent injection in power plant[J]. Chem Ind Eng Prog, 2018, 37(11):4460-4467. http://d.old.wanfangdata.com.cn/Periodical/hgjz201811044 [4] YANG Z Q, LI H L, YANG J P, FENG S H, LIU X, ZHAO J X, QU W Q, LI P, FENG Y, LEE P H, SHIH K. Nanosized copper selenide functionalized zeolitic imidazolate framework-8(CuSe/ZIF-8) for efficient immobilization of gas-phase elemental mercury[J]. Adv Funct Mater, 2019, 29(17):1807191. doi: 10.1002/adfm.201807191 [5] 王鹏鹰, 苏胜, 向军, 曹蕃, 尤默, 胡松, 孙路石, 张良平.低温SCR催化剂脱硝脱汞实验研究[J].燃烧科学与技术, 2014, 20(5):423-427. http://d.old.wanfangdata.com.cn/Periodical/rskxyjs201405009WANG Peng-ying, SU Sheng, XIANG Jun, CAO Fan, YOU Mo, HU Song, SUN Lu-shi, ZHANG Liang-ping. Experimental study on NO reduction and Hg0 oxidation over low temperature SCR catalyst[J]. J Combust Sci Technol, 2014, 20(5):423-427. http://d.old.wanfangdata.com.cn/Periodical/rskxyjs201405009 [6] 池桂龙, 沈伯雄, 朱少文, 何川.改性SCR催化剂对单质汞氧化性能的研究[J].燃料化学学报, 2016, 44(6):763-768. doi: 10.3969/j.issn.0253-2409.2016.06.018CHI Gui-long, SHEN Bo-xiong, ZHU Shao-wen, HE Chuan. Oxidation of elemental mercury over modified SCR catalysts[J]. J Fuel Chem Technol, 2016, 44(6):763-768. doi: 10.3969/j.issn.0253-2409.2016.06.018 [7] 赵莉, 何青松, 李琳, 陆强, 董长青, 杨勇平.改性SCR催化剂对Hg0催化氧化性能的研究[J].燃料化学学报, 2015, 43(5):628-634. doi: 10.3969/j.issn.0253-2409.2015.05.016ZHAO Li, HE Qing-song, LI Lin, LU Qiang, DONG Chang-qing, YANG Yong-ping. Research on the catalytic oxidation of Hg0 by modified SCR catalysts[J]. J Fuel Chem Technol, 2015, 43(5):628-634. doi: 10.3969/j.issn.0253-2409.2015.05.016 [8] 耿新泽, 段钰锋, 胡鹏, 柳帅, 梁财. SCR气氛下Ce-W/TiO2催化剂的脱硝协同脱汞特性[J].中国环境科学, 2019, 39(4):1419-1426. doi: 10.3969/j.issn.1000-6923.2019.04.009GENG Xin-ze, DUAN Yu-feng, HU Peng, LIU Shuai, LIANG Cai. Characteristics of denitrification and mercury removal of Ce-W/TiO2 catalysts in SCR atmosphere[J]. China Environ Sci, 2019, 39(4):1419-1426. doi: 10.3969/j.issn.1000-6923.2019.04.009 [9] ZHANG S B, ZHAO Y C, WANG Z H, ZHANG J Y, WANG L L, ZHENG C G. Integrated removal of NO and mercury from coal combustion flue gas using manganese oxides supported on TiO2[J]. J Environ Sci, 2017, 53:141-150. doi: 10.1016/j.jes.2015.10.038 [10] 于贤群, 鲍静静, 姜小祥, 杨宏旻. Mn-TiO2催化剂烟气脱汞性能及反应机理[J].中国电机工程学报, 2015, 35(13):3331-3337. http://d.old.wanfangdata.com.cn/Periodical/zgdjgcxb201513016YU Xian-qun, BAO Jing-jing, JIANG Xiao-xiang, YANG Hong-min. Performance and mechanism of catalytic oxidation for mercury by Mn-doped TiO2 catalysts in flue gas[J]. Proc CSEE, 2015, 35(13):3331-3337. http://d.old.wanfangdata.com.cn/Periodical/zgdjgcxb201513016 [11] LIU J, GUO R T, SUN X, PAN W G, WANG Z Y, LIU X Y, SHI X, QIN H, HU C X. Simultaneous NO reduction and Hg0 oxidation over Sb modified Mn/TiO2 catalyst[J]. Mater Chem Phys, 2019, 232:88-98. doi: 10.1016/j.matchemphys.2019.04.061 [12] ZHANG S B, ZHAO Y C, YANG J P, ZHANG J Y, ZHENG C G. Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas[J]. Chem Eng J, 2018, 348:618-629. doi: 10.1016/j.cej.2018.05.037 [13] ZHANG Y P, GUO W Q, WANG L F, SONG M, YANG L J, SHEN K, XU H T, ZHOU C C. Characterization and activity of V2O5-CeO2/TiO2-ZrO2 catalysts for NH3-selective catalytic reduction of NOx[J]. Chin J Catal, 2015, 36(10):1701-1710. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201510008 [14] ZHANG J Y, LI C T, ZHAO L Q, WANG T, LI S H, ZENG G M. A sol-gel Ti-Al-Ce-nanoparticle catalyst for simultaneous removal of NO and Hg0 from simulated flue gas[J]. Chem Eng J, 2017, 313:1535-1547. doi: 10.1016/j.cej.2016.11.039 [15] ITO K, KAKINO S C, IKEUE K T, MACHIDA M. NO adsorption/desorption property of TiO2-ZrO2 having tolerance to SO2 poisoning[J]. Appl Catal B:Environ, 2007, 74(1):137-143. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=03c53d00ff724df367eb523d54c61198 [16] ZHANG L, LI L L, CAO Y, XIONG Y, WU S G, SUN J F, TANG C J, GAO F, DONG L. Promotional effect of doping SnO2 into TiO2 over a CeO2/TiO2 catalyst for selective catalytic reduction of NO by NH3[J]. Catal Sci Technol, 2015, 5(4):2188-2196. doi: 10.1039/C4CY01412J [17] ZHANG Y P, HUANG T J, XIAO R, XU H T, ZHOU C C. A comparative study on the Mn/TiO2-M(M=Sn, Zr or Al) Ox catalysts for NH3-SCR reaction at low temperature[J]. Environ Technol, 2017, 39(10):1-28. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1080/21622515.2017.1329345 [18] 李鹏, 张亚平, 肖睿, 沈凯, 孙克勤, 徐海涛, 周长城.整体式V2O5-WO3/TiO2-ZrO2催化剂用于NH3选择性催化还原NOx[J].中南大学学报(自然科学版), 2013, 44(4):1719-1726. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zngydxxb201304061LI Peng, ZHANG Ya-ping, XIAO Rui, SHEN Kai, SUN Ke-qin, XU Hai-tao, ZHOU Chang-cheng. Selective catalytic reduction of NOx with NH3 over V2O5-WO3/TiO2-ZrO2 monolith catalysts[J]. J Cent South Univ (Sci Technol), 2013, 44(4):1719-1726. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zngydxxb201304061 [19] 李娟, 张亚平, 王文选, 王龙飞, 郭婉秋, 杨林军, 沈凯. TiO2-SnO2基钨催化剂的表面性质和NH3吸附特性及脱硝机理[J].东南大学学报(自然科学版), 2016, 46(1):92-99. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dndxxb201601016LI Juan, ZHANG Ya-ping, WANG Wen-xuan, WANG Long-fei, GUO Wan-qiu, YANG Lin-jun, SHEN Kai. Surface properties, NH3 adsorption characteristic and NOx removal mechanism of TiO2-SnO2 supported WO3 catalysts[J]. J Sout Univ (Nat Sci), 2016, 46(1):92-99. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dndxxb201601016 [20] LIU J, LI X Y, ZHAO Q D, HAO C, WANG S B, TADÉ M. Combined spectroscopic and theoretical approach to sulfur-poisoning on Cu-Supported Ti-Zr mixed oxide catalyst in the selective catalytic reduction of NO[J]. ACS Catal, 2014, 4(8):2426-2436. doi: 10.1021/cs5005739 [21] CHEN L, YAO X J, CAO J, YANG F M, TANG C J. DONG L. Effect of Ti4+ and Sn4+ co-incorporation on the catalytic performance of CeO2-MnOx catalyst for low temperature NH3-SCR[J]. Appl Surf Sci, 2019, 476: 283-292. [22] XIE J K, QU Z, YAN N Q, YANG S J, CHEN W M, HU L G, HUANG W J, LIU P. Novel regenerable sorbent based on Zr-Mn binary metal oxides for flue gas mercury retention and recovery[J]. J Hazard Mater, 2013, 261:206-213. doi: 10.1016/j.jhazmat.2013.07.027 [23] ZHAO L K, LI C T, LI S H, WANG Y, ZHANG J Y, WANG T, ZENG G M. Simultaneous removal of elemental mercury and NO in simulated flue gas over V2O5/ZrO2-CeO2 catalyst[J]. Appl Catal B:Environ, 2016, 198:420-430. doi: 10.1016/j.apcatb.2016.05.079 [24] XU H M, XIE J K, MA Y P, QU Z, ZHAO S J, CHEN W M, HUANG W J, YAN N Q. The cooperation of FeSn in a MnOx complex sorbent used for capturing elemental mercury[J]. Fuel, 2015, 140:803-809. doi: 10.1016/j.fuel.2014.10.004 [25] XU H M, QU Z, ZHAO S J, YUE D T, HUANG W J, YAN N Q. Enhancement of heterogeneous oxidation and adsorption of Hg0 in a wide temperature window using SnO2 supported LaMnO3 perovskite oxide[J]. Chem Eng J, 2016, 292:123-129. doi: 10.1016/j.cej.2016.01.078 [26] DONG L H, TANG Y X, LI B, ZHOU L Y, GONG F Z, HE H X, SUN B Z, TANG C J, GAO F, DONG L. Influence of molar ratio and calcination temperature on the properties of TixSn1-xO2 supporting copper oxide for CO oxidation[J].Appl Catal B:Environ, 2016, 180:451-462. doi: 10.1016/j.apcatb.2015.06.034 [27] YANG Y, LI H, ZHAO H T, QU R Y, ZHANG S, HU W S, YU X N, ZHU X B, LIU S J, ZHENG C H, GAO X. Structure and crystal phase transition effect of Sn doping on anatase TiO2 for dichloromethane decomposition[J]. J Hazard Mater, 2019, 371:156-164. doi: 10.1016/j.jhazmat.2019.02.103 [28] GÁLVEZ-LÓPEZ M F, MUÑOZ-BATISTA M J, ALVARADO-BELTRÁNA C G, ALMARAL-SÁNCHEZ J L, BACHILLER-BAEZA B, KUBACKA A, FERMÁNDEZ-GARCÍAB M. Sn modification of TiO2 anatase and rutile type phases:2-Propanol photo-oxidation under UV and visible light[J]. Appl Catal B:Environ, 2018, 228:130-141. doi: 10.1016/j.apcatb.2018.01.075 [29] YANG W, LIU Y X, WANG Q, PAN J F. Removal of elemental mercury from flue gas using wheat straw chars modified by Mn-Ce mixed oxides with ultrasonic-assisted impregnation[J]. Chem Eng J, 2017, 326:169-181. doi: 10.1016/j.cej.2017.05.106 [30] 张华伟, 陈江艳, 赵可, 牛庆欣, 王力. Mn/Ce掺杂改性半焦对模拟煤气中单质汞的脱除性能研究[J].燃料化学学报, 2016, 44(4):394-400. doi: 10.3969/j.issn.0253-2409.2016.04.002ZHANG Hua-wei, CHEN Jiang-yan, ZHAO Ke, NIU Qin-xin, WANG Li. Removal of vapor-phase elemental mercury from simulated syngas using semi-coke modified by Mn/Ce doping[J]. J Fuel Chem Technol, 2016, 44(4):394-400. doi: 10.3969/j.issn.0253-2409.2016.04.002 [31] ZHOU Z J, LIU X W, LI C P, ELVIS C X K, XU M H. Seawater-assisted synthesis of MnCe/zeolite-13X for removing elemental mercury from coal-fired flue gas[J]. Fuel, 2020, 262:116605. doi: 10.1016/j.fuel.2019.116605 [32] ZHANG A C, ZHANG Z H, CHEN J J, SHENG W, SUN L S, XIANG J. Effect of calcination temperature on the activity and structure of MnOx/TiO2 adsorbent for Hg0 removal[J]. Fuel Process Technol, 2015, 135:25-33. doi: 10.1016/j.fuproc.2014.10.007 [33] JIA B H, GUO J X, LUO H D, SHU S, FANG N J, LI J J. Study of NO removal and resistance to SO2 and H2O of MnOx/TiO2, MnOx/ZrO2 and MnOx/ZrO2-TiO2[J]. Appl Catal A:Gen, 2018, 553:82-90. doi: 10.1016/j.apcata.2017.12.016 [34] SHAN Y, YANG W, LI Y, LIU Y X, PAN J F. Preparation of microwave-activated magnetic bio-char adsorbent and study on removal of elemental mercury from flue gas[J]. Sci Total Environ, 2019, 697:134049. doi: 10.1016/j.scitotenv.2019.134049 [35] ZHAO H T, YANG G, GAO X, PANG C H, KINGMAN S W, WU T. Hg0 capture over CoMoS/γ-Al2O3 with MoS2 nanosheets at low temperatures[J]. Environ Sci Technol, 2016, 50(2):1056-1064. doi: 10.1021/acs.est.5b04278 [36] ZHAO H T, EZEH C I, YIN S F, XIE Z L, PANG C H, ZHENG C H, GAO X, WU T. MoO3-adjusted δ-MnO2 nanosheet for catalytic oxidation of Hg0 to Hg2+[J].Appl Catal B:Environ, 2020, 263:117829. doi: 10.1016/j.apcatb.2019.117829 [37] LI J H, CHEN J J, KE R, LUO C K, HAO J M. Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts[J]. Catal Commun, 2007, 8(12):1896-1900. doi: 10.1016/j.catcom.2007.03.007 [38] WANG S X, GUO R T, PAN W G, CHEN Q L, SUN P, LI M Y, LIU S M. The deactivation of Ce/TiO2 catalyst for NH3-SCR reaction by alkali metals:TPD and DRIFT studies[J]. Catal Commun, 2017, 89:143-147. doi: 10.1016/j.catcom.2016.11.005 [39] SUN P, GUO R T, LIU S M, WANG S X, PAN W G, LI M Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu[J]. Appl Catal A:Gen, 2017, 531:129-138. doi: 10.1016/j.apcata.2016.10.027 [40] WU Z B, JIANG B Q, LIU Y, ZHAO W R, GUAN B H. Experimental study on a low-temperature SCR catalyst based on MnOx/TiO2 prepared by sol-gel method[J]. J Hazard Mater, 2007, 145(3):488-494. doi: 10.1016/j.jhazmat.2006.11.045 [41] SMIRNIOTIS P G, SREEKANTH P M, PEÑA D A, JENKINS R G. Manganese oxide catalysts supported on TiO2, Al2O3, and SiO2:A comparison for low-temperature SCR of NO with NH3[J]. Ind Eng Chem Res, 2006, 45(19):6436-6443. doi: 10.1021/ie060484t [42] DONG L, HUANG Y J, CHEN H, LIU L Q, LIU C Q, XU L G, ZHA J R, WANG Y X, LIU H. Magnetic γ-Fe2O3-loaded attapulgite sorbent for Hg0 removal in coal-fired flue gas[J]. Energy Fuels, 2019, 33(8):7522-7533. doi: 10.1021/acs.energyfuels.9b01136 [43] LIU Z Y, YANG W, XU W, LIU Y X. Removal of elemental mercury by bio-chars derived from seaweed impregnated with potassium iodine[J]. Chem Eng J, 2018, 339:468-478. doi: 10.1016/j.cej.2018.01.148 [44] LIU D J, ZHOU W G, WU J. Effect of Ce and La on the activity of CuO/ZSM-5 and MnOx/ZSM-5 composites for elemental mercury removal at low temperature[J]. Fuel, 2017, 194:115-122. doi: 10.1016/j.fuel.2016.12.076 [45] XU Y L, ZHONG Q, LIU X Y. Elemental mercury oxidation and adsorption on magnesite powder modified by Mn at low temperature[J]. J Hazard Mater, 2015, 283:252-259. doi: 10.1016/j.jhazmat.2014.09.034 [46] HE J, REDDY G K, THIEL S W, SMIRNIOTIS P G, PINTO N G. Ceria-modified manganese oxide/titania materials for removal of elemental and oxidized mercury from flue gas[J]. J Phys Chem C, 2011, 115(49):24300-24309. doi: 10.1021/jp208768p -





 下载:
下载: