Performance of Mn-Ce co-doped siderite catalysts in the selective catalytic reduction of NOx by NH3
-
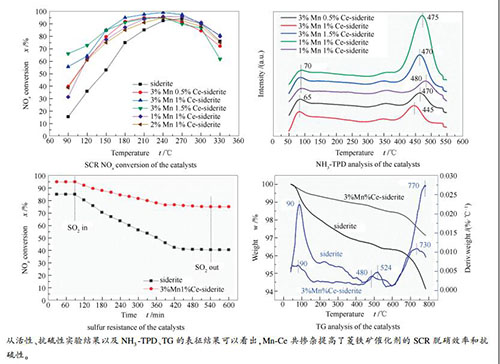
摘要: 富含过渡元素的菱铁矿是用于制备选择性催化还原(SCR)脱硝催化剂的理想材料。在本研究中,对菱铁矿掺杂了Mn和Ce,并研究了Mn-Ce共掺杂改性菱铁矿在NH3-SCR反应中去除NOx的活性。结果表明,经过450℃煅烧后菱铁矿的主要成分FeCO3能够转化为Fe2O3。菱铁矿掺杂Mn和Ce后能够提高比表面积和表面酸度,降低硫酸铵盐在催化剂表面上的热稳定性。因此,Mn-Ce共掺杂改性菱铁矿催化剂表现出较高的SCR脱硝活性和抗硫性。3% Mn1% Ce-菱铁矿催化剂在脱硝效率高于90%的温度窗口能够拓宽至180-300℃,同时在引入SO2 7.5 h后该催化剂的脱硝效率仍高于75%。Abstract: Siderite, rich in the transition elements, is an idea material to prepare the catalysts for the selective catalytic reduction (SCR) of NOx by NH3. In this work, siderite was doped with Mn and Ce and the performance of Mn-Ce co-doped siderite catalysts in the removal of NOx (de-NOx) by SCR with NH3 was then investigated. The results illustrate that FeCO3 as the main component of siderite can be converted into Fe2O3 by calcination at 450℃. The doping of siderite with Mn and Ce can enhance the surface area and acidity of siderite and reduce the thermal stability of ammonium sulfate formed on the catalyst surface. As a result, the Mn-Ce co-doped siderite catalysts exhibit high efficiency in the de-NOx by SCR and high resistance against sulfur. Over the 3%Mn1%Ce-siderite catalyst, high NOx conversion (>90%) is achieved in the temperature window of 180-300℃; moreover, the NOx conversion remains above 75% even after introducing SO2 for 7.5 h.
-
Key words:
- Mn-Ce co-doping /
- siderite /
- selective catalytic reduction /
- de-NOx /
- sulfur resistance
-
Table 1 Textural properties of the siderite catalysts calcined at different temperatures
Calc. temperature t/℃ Surface area A/(m2·g-1) Pore volume v /(cm3·g-1) Pore size d/nm Uncalcined 21.5 0.025 4.7 400 42.8 0.055 4.8 450 64.8 0.138 9.7 500 58.4 0.133 9.1 Table 2 XRF analysis results of various Mn-modified siderite catalysts
Sample Composition w/% Fe Mn Si Al Mg Siderite 44.25 2.941 1.053 0.2834 0.4112 1%Mn-siderite 43.69 3.953 0.9820 0.2152 0.7329 2%Mn-siderite 42.94 5.074 0.9240 0.1978 0.6765 3%Mn-siderite 42.19 5.981 0.9060 0.1939 0.4534 Table 3 Textural properties of various Mn-modified siderite catalysts
Sample Surface area A/(m2·g-1) Pore volume v /(cm3·g-1) Pore size d /nm Siderite 64.8 0.138 9.7 1%Mn-siderite 68.4 0.133 8.7 2%Mn-siderite 71.0 0.146 8.2 3%Mn-siderite 73.2 0.137 7.5 Table 4 Experimental group setting for the investigation of the effect of Mn and Ce co-doping on the performance of modified siderite catalyst
Test number Mn doping amount /% Ce doping amount /% 1 0 0 2 3.0 0.5 3 3.0 1.0 4 3.0 1.5 5 1.0 1.0 6 2.0 1.0 -
[1] BUSCA G, LIETTI L, RAMIS G, BERTI F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review[J]. Appl Catal B: Environ, 1998, 18(1/2): 1-36. [2] FORZATTI P. Environmental catalysis for stationary applications[J]. Catal Today, 2000, 62(1): 51-65. [3] YANG S, WANG C, LI J, YAN N, MA L, CHANG H. Low temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel: performance, mechanism and kinetic study[J]. Appl Catal B: Environ, 2011, 110: 71-80. doi: 10.1016/j.apcatb.2011.08.027 [4] LIU F, HE H, ZHANG C, SHAN W, SHI X. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst[J]. Catal Today, 2010, 175(1): 18-25. [5] LIU C, YANG S, MA L, PENG Y, HAMIDREZA A, CHANG H, LI J. Comparison on the performance of α-Fe2O3 and γ-Fe2O3 for selective catalytic reduction of nitrogen oxides with ammonia[J]. Catal Lett, 2013, 143(7): 697-704. doi: 10.1007/s10562-013-1017-3 [6] XIE J, FANG D, HE F, CHEN J, FU Z, CHEN X. Performance and mechanism about MnOx species included in MnOx/TiO2 catalysts for SCR at low temperature[J]. Catal Commun, 2012, 28: 77-81. doi: 10.1016/j.catcom.2012.08.022 [7] JIANG B Q, LIU Y, WU Z B. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods[J]. J Hazard Mater, 2009, 162(2/3): 1249-1254. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=59f5891610eddf7a31915712c2a7491d [8] ELLMERS I, VELEZ R P, BENTRUP U, BRUCKNER A, GUNERT W. Oxidation and selective reduction of NO over Fe-ZSM-5-How related are these reactions?[J]. J Catal, 2014, 311: 199-211. doi: 10.1016/j.jcat.2013.11.024 [9] YAO G H, GUI K T, WANG F. Low-temperature De-NOx by selective catalytic reduction based on iron-based catalysts[J]. Chem Eng Technol, 2010, 33(7): 1093-1098. doi: 10.1002/ceat.201000015 [10] WU Z, JIANG B, LIU Y, ZHAO W, GUAN B. Experimental study on a low-temperature SCR catalyst based on MnOx/TiO2 prepared by so-gel method[J]. J Hazard Mater, 2007, 145(3): 488-494. doi: 10.1016/j.jhazmat.2006.11.045 [11] JIA B, GUO J, LUO H, SHU S, FANG N, LI J. Study of NO removal and resistance to SO2 and H2O of MnOx/TiO2, MnOx/ZrO2 and MnOx/ZrO2-TiO2[J]. Appl Catal A: Gen, 2018, 553: 82-90. doi: 10.1016/j.apcata.2017.12.016 [12] GUO R, LI M, SUN P, PAN W, LIU S, LIU J, SUN X, LIU S. Mechanistic investigation of the promotion effect of Bi modification on the NH3-SCR performance of Ce/TiO2 catalyst[J]. J Phys Chem C, 2017, 121(49): 27535-27545. doi: 10.1021/acs.jpcc.7b10342 [13] XU L, LI X S, CROCKER M, ZHANG Z S, ZHU A M, SHI C. A study of the mechanism of low-temperature SCR of NO with NH3 on MnOx/CeO2[J]. J Mol Catal A: Chem, 2013, 378: 82-90. doi: 10.1016/j.molcata.2013.05.021 [14] ZHAO W, TANG Y, WAN Y, LI L, YAO S, LI X, GU J, LI Y, SHI J. Promotion effects of SiO2 or/and Al2O3 doped CeO2/TiO2 catalysts for selective catalytic reduction of NO by NH3[J]. J Hazard Mater, 2014, 278: 350-359. doi: 10.1016/j.jhazmat.2014.05.071 [15] DU X, WANG X, CHEN Y, GAO X, ZHANG L. Supported mental sulfates on Ce-TiOx as catalysts for NH3-SCR of NO: High resistances to SO2 and potassium[J]. J Ind Eng Chem, 2016, 36: 271-278. doi: 10.1016/j.jiec.2016.02.013 [16] YE D, REN X, QU R, LIU S, ZHENG C, GAO X. Designing SO2-resistant cerium-based catalyst by modifying with Fe2O3 for the selective catalytic reduction of NO with NH3[J]. J Mol Catal, 2019, 462: 10-18. doi: 10.1016/j.mcat.2018.10.007 [17] JIN R, LIU Y, WANG Y, CEN W, WU Z, WANG H, WENG X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature[J]. Appl Catal B: Environ, 2014, 148/149: 582-588. doi: 10.1016/j.apcatb.2013.09.016 [18] HUANG L, LI X, HUA J. Effect of Sn doping on denitrification and sulfur resistance performance of Ce-Mn/AC catalyst[J]. Adv Eng Sci, 2019, 51(5): 185-191. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=scdxxb-gckx201905023 [19] ZHAO S, LIU L, WANG J, XIONG H. Effect of Fe, Ce and Cu on low temperature denitrification and sulphur resistance of Mn/AC catalysts[J]. Appl Chem Ind, 2019, 48(9): 2107-2112. [20] WU Y, LIANG H, ZHAO C, CHEN X, CHEN C, DAI C, TANG J. Effect of support on low temperature denitrification performance of Mn-Ce catalysts[J]. Pet Process Petrochem, 2019, 50(40): 44-48. http://d.old.wanfangdata.com.cn/Periodical/sylzyhg201904010 [21] ZHANG L, LI L, CAO Y, YAO X, GE C, GAO F, DENG Y, TANG C, DONG L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3[J]. Appl Catal B: Environ, 2015, 165: 589-598. doi: 10.1016/j.apcatb.2014.10.029 [22] JIN R, YUE L, WU Z, WANG H, GU T. Low-temperature selective catalytic reduction of NO with NH3 over Mn-Ce oxides supported on TiO2 and Al2O3: A comparative study[J]. Chemosphere, 2010, 78(9): 1160-1166. doi: 10.1016/j.chemosphere.2009.11.049 [23] XU W, HE H, YU Y. Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3[J]. J Phys Chem C, 2009, 113(11): 4426-4432. doi: 10.1021/jp8088148 [24] WU Z, JIN R, LIU Y, WANG H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature[J]. Catal Commun, 2008, 9(13): 2217-2220. doi: 10.1016/j.catcom.2008.05.001 [25] KIJLSTRA W S, BIERVLIET M, POELS E K, BLIEK A. Deactivation by SO2 of MnOx/Al2O3 catalysts used for the selective catalytic reduction of NO with NH3 at low temperatures[J]. Appl Catal B: Environ, 1998, 16(4): 327-337. doi: 10.1016/S0926-3373(97)00089-1 [26] ZHU Z, NIU H, LIU Z, LIU S. Decomposition and reactivity of NH4HSO4 on V2O5/AC catalysts used for NO reduction with ammonia[J]. J Catal, 2000, 195(2): 268-278. doi: 10.1006/jcat.2000.2961 -





 下载:
下载: