-
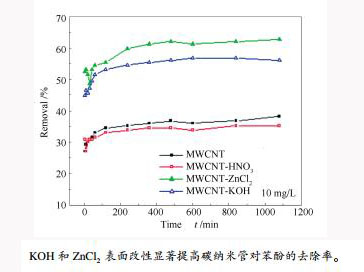
摘要: 通过扫描电子显微镜、X射线衍射仪、N2吸附分析仪及Boehm滴定法获得ZnCl2、KOH和HNO3化学处理对高纯多壁碳纳米管的结构和表面含氧官能团的影响,通过批处理实验考察吸附条件(吸附时间、初始浓度、温度)对处理前后的碳纳米管吸附苯酚行为的影响,并采用准一级、准二级、Evolich动力学模型和热力学方程拟合其吸附数据,分析其动力学行为、热力学行为和吸附机理。结果表明,虽然ZnCl2、KOH和HNO3化学处理法均未对碳纳米管BET比表面积产生显著影响,但会影响其表面化学性质(即,对于ZnCl2和KOH化学处理降低表面羧基、内酯基含量和增大碱性官能团量,而对于HNO3化学处理可以增大表面羧基、内酯基含量,而碱性官能团略有增加);改性处理影响碳纳米管去除苯酚效率:由于ZnCl2和KOH改性处理降低碳纳米管表面羧基量,故其提高了苯酚去除率,而HNO3处理则略减小碳纳米管的苯酚去除率,可能是由于碳纳米管结构和表面化学性质共同影响所致;碳纳米管的苯酚去除率均随苯酚溶液初始浓度的增大而减小;高温不利于吸附;热力学研究发现碳纳米管吸附苯酚过程是自发的和放热的,属于物理吸附;动力学研究表明,吸附过程符合准二级动力学方程。通过ZnCl2和KOH化学处理,可以显著提高碳纳米管对苯酚的吸附性能。Abstract: The texture and surface chemistry of carbon nanotubes before and after chemical treatment using ZnCl2, KOH and HNO3 were determined by scanning electronic microscope, X-ray diffraction, N2 adsorption, and Boehm titration; and the effect of adsorptive conditions (contact time, initial concentration and temperature) on phenol removal and the thermodynamic and kinetic behavior and adsorption mechanism were investigated by tests and data fitting with three kinetic models (pseudo-first order, pseudo-second order and the Elovich kinetic equations) as well as thermodynamic equation. The results show that the treatment by HNO3, ZnCl2 or KOH less changes the BET surface area of carbon nanotubes, but obviously changes the surface chemical property. Specifically, the treatment by HNO3 obviously enhances surface acidic groups and slightly increases basic groups, whereas the treatment by ZnCl2 or KOH greatly decreases surface carboxyl groups and lactonic groups but obviously increases surface basic groups, which affects the phenol removal by carbon nanotubes. It is found that the phenol removal by carbon nanotubes treated with ZnCl2 or KOH increases due to a decrease in surface carboxyl groups of carbon nanotubes, but HNO3 treatment slightly reduces the phenol removal possibly because the adsorption is influenced by both structure and surface chemical property. Moreover, the adsorption of phenol by carbon nanotubes is spontaneous, exothermic and physically controlled, and the adsorption process of phenol by carbon nanotubes complies with the pseudo-second order equation.
-
Key words:
- carbon nanotube /
- chemical treatment /
- phenol /
- adsorption /
- modeling
-
表 1 吸附剂的孔性质
Table 1 Textural properties of adsorbents
Adsorbent ABET /(m2·g-1) vmic /(cm3·g-1) vmeso /(cm3·g-1) Amic /(m2·g-1) d /nm MWCNT 451 0.015 0.674 5.94 5.91 MWCNT-HNO3 458 0.0033 0.560 10.2 4.85 MWCNT-ZnCl2 466 0.013 0.561 32.9 4.87 MWCNT-KOH 469 0.001 0.650 6.46 5.47 note: d is the mean pore width (4v/A by BET) 表 2 采用Boehm滴定法测定的吸附剂表面官能团含量
Table 2 Surface groups of adsorbents by Boehm titration
Adsorbent Acidic groups
/(mmol·g-1)Carboxyl
/(mmol·g-1)Lactonic groups
/(mmol·g-1)Phenolic groups
/(mmol·g-1)Basic groups
/(mmol·g-1)pHpzc MWCNT 2.163 2.044 0.105 0.014 2.015 6.03 MWCNT-HNO3 2.828 2.519 0.180 0.129 2.150 6.01 MWCNT-ZnCl2 1.688 1.331 0.018 0.339 2.563 6.04 MWCNT-KOH 1.925 1.569 0.035 0.321 2.618 6.05 表 3 吸附剂吸附10 mg/L苯酚的去除率
Table 3 Removal percentage of phenol by adsorbents
Adsorbent MWCNT MWCNT-HNO3 MWCNT-ZnCl2 MWCNT-KOH Removal η/% 37.60 35.59 63.06 57.95 表 4 吸附剂吸附苯酚的动力学模型拟合参数
Table 4 Fitting parameters of adsorption of phenol onto adsorbents by the kinetic models
Model MWCNT MWCNT-HNO3 MWCNT-ZnCl MWCNT-KOH Pseudo-first order k1/min-1 0.345 0.507 0.469 0.345 qe /(mg·g-1) 10.8 6.75 11.7 10.8 R2 0.936 0.939 0.923 0.936 Pseudo-second order k2 /(×10-3 ·g·mg-1·min-1) 57.7 145 79.8 57.7 qe /(mg·g-1) 11.1 6.92 12.0 11.1 R2 0.968 0.963 0.944 0.968 Elovich equation αa /(mg·g-1·min-1) 93.5 91.9 122 93.5 βd /(g·mg-1) 0.885 1.48 0.828 0.885 R2 0.707 0.649 0.677 0.707 表 5 吸附剂吸附苯酚的热力学参数
Table 5 Thermodynamic parameters of adsorption of phenol onto adsorbents
Adsorbent 303.15 K 313.15 K 323.15 K 333.15 K ΔG°/(kJ·mol-1) MWCNT -1.123 -1.001 -0.951 -0.637 MWCNT-HNO3 -0.892 -0.761 -0.701 -0.549 MWCNT-ZnCl2 -3.194 -3.133 -3.148 -3.158 MWCNT-KOH -2.331 -2.251 -2.162 -2.146 ΔH°/(kJ·mol-1) MWCNT -5.653 MWCNT-HNO3 -4.183 MWCNT-ZnCl2 -3.488 MWCNT-KOH -4.298 ΔS°/(J·K-1·mol-1) MWCNT -14.852 MWCNT-HNO3 -10.866 MWCNT-ZnCl2 -1.037 MWCNT-KOH -6.524 R2 MWCNT 0.958 MWCNT-HNO3 0.992 MWCNT-ZnCl2 0.986 MWCNT-KOH 0.993 -
[1] PRUDEN B B, LE H. Wet air oxidation of soluble components in wastewater[J]. Can J Chem Eng, 1976, 54(4):319-325. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1002/cjce.5450540413 [2] BANAT F, ALBASHIR B, ALASHEH S, HAYAJNEH O. Adsorption of phenol by bentonite[J]. Environ Pollut, 2000, 107(3):391-398. http://d.old.wanfangdata.com.cn/Periodical/hjkx201205038 [3] LORENCGRABOWSKA E, DIEZ M A, GRYGLEWICZ G. Influence of pore size distribution on the adsorption of phenol on PET-based activated carbons[J]. J Colloid Interf Sci, 2016, 469:205-212. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=24963c8a943c2d36f2903f7b069c4def [4] GORBACH A, STEGMAIER M, EIGENBERGER G. Measurement and modeling of water vapor adsorption on zeolite 4A-equilibria and kinetics[J]. Adsorption, 2004, 10(1):29-46. http://cn.bing.com/academic/profile?id=aeb9198f454c1d1db7f7896631dae9b9&encoded=0&v=paper_preview&mkt=zh-cn [5] LI W, YAN J, YAN Z, SONG Y, JIAO W, QI G, LIU Y. Adsorption of phenol by activated carbon in rotating packed bed:Experiment and modeling[J]. Appl Therm Eng, 2018, 142:760-766. http://cn.bing.com/academic/profile?id=7e201e60abbc4ae9284c80424b932aee&encoded=0&v=paper_preview&mkt=zh-cn [6] FIERRO V, TORNÉ-FERNÁNDEZ V, MONTANÉ D, CELZARD A. Adsorption of phenol onto activated carbons having different textural and surface properties[J]. Microporous Mesorporpus Mater, 2008, 111(1/3):276-284. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2bfa6f8faf06a187361a383310f73142 [7] LI B, SUN K, GUO Y, TIAN J, XUE Y, SUN D. Adsorption kinetics of phenol from water on Fe/AC[J]. Fuel, 2013, 110:99-106. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dd17a3f1a764afd4134637ed326cde32 [8] LI B, LEI Z, ZHANG X, HUANG Z. Adsorption of simple aromatics from aqueous solutions on modified activated carbon fiber[J]. Catal Today, 2010, 158(3/4):515-520. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dad2564d6e9b147a3afe6bbd579b19d6 [9] 张雪, 崔春月, 杨文方, 王婧, 王颖.不同微结构碳纳米管对五氯苯酚的吸附和再生[J].水处理技术, 2019, 45(11):50-54.ZHANG Xue, CHUI Chun-yue, YANG Wen-fang, WANG Jin, WANG Ying. Adsorption and regeneration of pentachlorophenol by different microstructure carbon nanotubes[J]. Technol Water Treat, 2019, 45(11):50-54. [10] 姚夏妍, 鲁兴武, 程亮, 张恩玉.磁场对氨基和巯基修饰多壁碳纳米管复合材料吸附苯酚的影响[J].化工新型材料, 2019, 47(1):189-193. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxxcl201901046YAO Xia-yan, LU Xing-wu, CHENG Liang, ZHANG En-yu. Influence of magnetic field on adsorption of phenol by NH2-SH-MWCNTs composite[J]. New Chem Mater, 2019, 47(1):189-193. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxxcl201901046 [11] 王可, 李虹雨, 任华堂, 夏建新, 邢璇, 张璟, 马忆波, 师蕾.多壁碳纳米管吸附水中典型苯酚类物质[J].工业水处理, 2018, 38(11):40-44. http://d.old.wanfangdata.com.cn/Periodical/gyscl201811009WANG Ke, LI Hong-yu, REN Hua-tang, XIA Jian-xin, XING Xuan, ZHANG Jing, MA Yi-bo, SHI Lei. Adsorption of MWCNTs for typical phenolic compounds in water[J]. Ind Water Treat, 2018, 38(11):40-44. http://d.old.wanfangdata.com.cn/Periodical/gyscl201811009 [12] 韩道丽, 赵元黎, 朱双美, 李华洋, 梁二军.浓硝酸处理碳纳米管对4-硝基苯酚的吸附性能研究[J].光散射学报, 2006, 18(4):319-322. http://d.old.wanfangdata.com.cn/Periodical/gssxb200604007HAN Dao-li, ZHAO Yuan-li, ZHU Shuang-mei, LI Hua-yang, LIANG Er-jun. Studies on adsorption of P-nitrophenol on multi-wall carbon nanotubes after concentrated nitric acid treatment[J]. J Light Scattering, 2006, 18(4):319-322. http://d.old.wanfangdata.com.cn/Periodical/gssxb200604007 [13] MATTSON J A, MARK J H B, MALBIN M D, WEBER J W J. Surface chemistry of active carbon:Specific adsorption of phenols[J]. J Colloid Interf Sci, 1969, 31(1):116-130. http://cn.bing.com/academic/profile?id=9f0a2887afcdada202dcd15a20d9b902&encoded=0&v=paper_preview&mkt=zh-cn [14] BOEHM H P. Some aspects of the surface chemistry of carbon blacks and other carbons[J]. Carbon, 1994, 32(5):759-769. doi: 10.1016-0008-6223(94)90031-0/ [15] SHARMA V K. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment-A Review[J]. J Environ Sci Health A Tox Hazard Subst Environ Eng, 2009, 44(14):1485-1495. http://cn.bing.com/academic/profile?id=3a1f95792d3b01a3369263e60654b2af&encoded=0&v=paper_preview&mkt=zh-cn [16] VILLACAÑAS F, PEREIRA M F R, ÓRFÃO J J M, FIGUEIREDO J L. Adsorption of simple aromatic compounds on activated carbons[J]. J Colloid Interface Sci, 2006, 293(1):128-136. http://d.old.wanfangdata.com.cn/Conference/WFHYXW651350 [17] JEAN-PIERRE S. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics[J]. Chem Eng J, 2016, 300:254-263. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9a2434c975298d12449231827fa20cd1 [18] HO Y S, MCKAY G. Pseudo-second order model for sorption processes[J]. Process Biochem, 1999, 34(5):451-465. doi: 10.1016-S0032-9592(98)00112-5/ [19] CHIEN S H, CLAYTON W R. Application of elovich equation to the kinetics of phosphate release and sorption in soils[J]. Soil Sci Soc Am J, 1980, 44(2):265-268. http://cn.bing.com/academic/profile?id=77dcd9f32ef47fbd398acea3bd1420df&encoded=0&v=paper_preview&mkt=zh-cn [20] 傅献彩, 沈文霞, 姚天扬.物理化学[M]. 5版.北京:高等教育出版社, 2006.FU Xian-cai, SHENG Wen-xia, YAO Tian-yang. Physical Chemistry[M]. 5th ed. Beijing:Higher Education Press, 2006. [21] 李爱民, 张全兴, 刘富强, 费正皓, 王学江, 陈金龙.一种亲水的酚羟基修饰聚苯乙烯树脂对苯酚类化合物的吸附热力学[J].离子交换与吸附, 2001, 17(6):515-525.LI Ai-min, ZHANG Quan-xing, LI Fu-qiang, FEI Zheng-hao, WANG Xu-jiang CHEN Jin-long. Thermodynamic study of adsorption of phenolic compounds on a phenol hydroxyl modified polystyrene[J]. Ion Exchange Adsorption, 2001, 17(6):515-525. [22] 段家铁, 徐满才, 李海涛, 史作清, 何炳林.氧化叔胺树脂的合成及其对苯酚的吸附性能研究[J].离子交换与吸附, 2004, 20(1):40-45. http://d.old.wanfangdata.com.cn/Periodical/lzjhyxf200401006DUAN Jia-tie, XU Man-cai, LI Hai-tao, SHI Zuo-qing, He Bing-lin. Synthesis of oxidized teritamine resin and its adsorption property for phenol[J]. Ion Exchange Adsorption, 2004, 20(1):40-45. http://d.old.wanfangdata.com.cn/Periodical/lzjhyxf200401006 -





 下载:
下载: