A comparison of MoS2 catalysts hydrothermally synthesized from different sulfur precursors in their morphology and hydrodeoxygenation activity
-
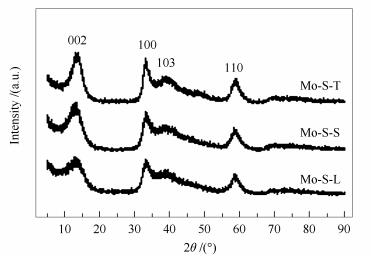
摘要: 分别采用硫脲、L-胱氨酸和硫磺为硫源水热合成了三种MoS2催化剂,对其结构和形貌特征进行了表征,并以对甲酚为探针化合物,比较研究了三种MoS2的加氢脱氧(HDO)催化活性。结果表明,硫源对MoS2晶体结构的影响不大,但对其形貌和比表面积影响较大。与商业MoS2相比,所制备的MoS2催化剂都表现出更高的HDO活性;其中,以硫脲为原料合成的MoS2具有较高的比表面积和花状结构,其催化活性最高,在300℃下进行对甲酚的HDO反应,脱氧度可达99.3%。Abstract: Three MoS2 catalysts were synthesized by a hydrothermal method using different sulfur precursors such as thiourea, L-cystine and sulfur powder; their differences in the structure, morphology, and catalytic activity in the hydrodeoxygenation (HDO) of p-cresol were comparatively investigated. The results illustrated that the sulfur source has a significant influence on the morphology and surface area of the as-synthesized MoS2 catalysts. All the hydrothermally synthesized MoS2 catalysts show much higher activity in HDO than the commercial MoS2 sample. Among three MoS2 catalysts, the one prepared from thiourea, with a high surface area and flower-like morphology, exhibits the highest activity in HDO; over it, a deoxygenation degree of 99.3% is achieved at 300℃.
-
Key words:
- MoS2 /
- hydrothermal synthesis /
- sulfur precursor /
- morphology /
- hydrodeoxygenation /
- p-cresol
-
Table 1 Reaction results for HDO of p-cresol on MoS2 catalysts at 275 and 300 ℃ for 8 h
Catalyst p-cresol conversion x/% Product distribution wm/% D.D./% 4-methylcyclohexene methylcyclohexane toluene 275 ℃ Mo-S-C 8.8 7.1 3.1 89.9 7.6 Mo-S-T 87.4 5.5 26.1 68.4 85.8 Mo-S-L 84.4 4.4 25.5 70.2 82.5 Mo-S-S 70.3 5.9 20.2 73.9 67.2 300 ℃ Mo-S-C 29.1 6.5 7.9 85.6 26.0 Mo-S-T 99.4 4.3 16.1 79.7 99.3 Mo-S-L 95.2 3.9 17.5 78.7 94.4 Mo-S-S 90.1 4.4 13.5 82.2 88.7 -
[1] LIU Z, GUAN D B, WEI W, DAVIS S J, CIAIS P, BAI J, PENG S S, ZHANG Q, HUBACEK K, MARLAND G, ANDRES R J, CRAWFORD-BROWN D, LIN J T, ZHAO H Y, HONG C P, BODEN T A, FENG K S, PETERS G P, XI F M, LIU J G, LI Y, ZHAO Y, ZENG N, HE K B. Reduced carbon emission estimates from fossil fuel combustion and cement production in China[J]. Nature, 2015, 524(7565):335-338. doi: 10.1038/nature14677 [2] LI C Z, ZHAO X C, WANG A Q, HUBER G W, ZHANG T. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chem Rev, 2015, 115(21):11559-11624. doi: 10.1021/acs.chemrev.5b00155 [3] SAIDI M, SAMIMI F, KARIMIPOURFARD D, NIMMANWUDIPONG T, GATES B C, RAHIMPOUR M R. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation[J]. Energy Environ Sci, 2014, 7(1):103-129. doi: 10.1039/C3EE43081B [4] PATEL M, KUMAR A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil:A review[J]. Renewable Sustainable Energy Rev, 2016, 58:1293-1307. doi: 10.1016/j.rser.2015.12.146 [5] GRILC M, LIKOZAR B, LEVEC J. Hydrodeoxygenation and hydrocracking of solvolysed lignocellulosic biomass by oxide, reduced and sulphide form of NiMo, Ni, Mo and Pd catalysts[J]. Appl Catal B:Environ, 2014, 150-151:275-287. doi: 10.1016/j.apcatb.2013.12.030 [6] SCHIMMING S M, LAMONT O D, KÖNIG M, ROGERS A K, D'AMICO A D, YUNG M M, SIEVERS C. Hydrodeoxygenation of guaiacol over ceria-zirconia catalysts[J]. ChemSusChem, 2015, 8(12):2073-2083. doi: 10.1002/cssc.201500317 [7] LUSKA K L, MIGOWSKI P, EL SAYED S, LEITNER W. Synergistic interaction within bifunctional ruthenium Nanoparticle/SILP catalysts for the selective hydrodeoxygenation of phenols[J]. Angew Chem Int Ed, 2015, 54(52):15750-15755. doi: 10.1002/anie.201508513 [8] WANG G H, CAO Z W, GU D, PFÄNDER N, SWERTZ A C, SPLIETHOFF B, BONGARD H-J, WEIDENTHALER C, SCHMIDT W, RINALDI R, SCHVTH F. Nitrogen-doped ordered mesoporous carbon supported bimetallic PtCo nanoparticles for upgrading of biophenolics[J]. Angew Chem Int Ed, 2016, 55(31):8850-8855. doi: 10.1002/anie.201511558 [9] SULLIVAN M M, CHEN C J, BHAN A. Catalytic deoxygenation on transition metal carbide catalysts[J]. Catal Sci Technol, 2016, 6(3):602-616. doi: 10.1039/C5CY01665G [10] WANG W Y, LIU P L, WU K, TAN S, LI W S, YANG Y Q. Preparation of hydrophobic reduced graphene oxide supported Ni-B-P-O and Co-B-P-O catalysts and their high hydrodeoxygenation activities[J]. Green Chem, 2016, 18(4):984-988. doi: 10.1039/C5GC02073E [11] GRILC M, VERYASOV G, LIKOZAR B, JESIH A, LEVEC J. Hydrodeoxygenation of solvolysed lignocellulosic biomass by unsupported MoS2, MoO2, Mo2C and WS2 catalysts[J]. Appl Catal B:Environ, 2015, 163(0):467-477. doi: 10.1007/s11705-017-1655-x [12] YANG Y Q, TYE C T, SMITH K J. Influence of MoS2 catalyst morphology on the hydrodeoxygenation of phenols[J]. Catal Commun, 2008, 9(6):1364-1368. doi: 10.1016/j.catcom.2007.11.035 [13] RUIZ P E, FREDERICK B G, DE SISTO W J, AUSTIN R N, RADOVIC L R, LEIVA K, GARCÍA R, ESCALONA N, WHEELER M C. Guaiacol hydrodeoxygenation on MoS2 catalysts:Influence of activated carbon supports[J]. Catal Commun, 2012, 27:44-48. doi: 10.1016/j.catcom.2012.06.021 [14] YOOSUK B, TUMNANTONG D, PRASASSARAKICH P. Unsupported MoS2 and CoMoS2 catalysts for hydrodeoxygenation of phenol[J]. Chem Eng Sci, 2012, 79:1-7. doi: 10.1016/j.ces.2012.05.020 [15] VERYASOV G, GRILC M, LIKOZAR B, JESIH A. Hydrodeoxygenation of liquefied biomass on urchin-like MoS2[J]. Catal Commun, 2014, 46:183-186. doi: 10.1016/j.catcom.2013.12.011 [16] ITTHIBENCHAPONG V, RATANATAWANATE C, OURA M, FAUNGNAWAKIJ K. A facile and low-cost synthesis of MoS2 for hydrodeoxygenation of phenol[J]. Catal Commun, 2015, 68:31-35. doi: 10.1016/j.catcom.2015.04.024 [17] LIU G L, MA H L, TEIXEIRA I, SUN Z Y, XIA Q N, HONG X L, TSANG S, CHI E. Hydrazine-assisted liquid exfoliation of MoS2 for catalytic hydrodeoxygenation of 4-methylphenol[J]. Chem Eur J, 2016, 22(9):2910-2914. doi: 10.1002/chem.201504009 [18] AMAYA S L, ALONSO-N ÚÑEZ G, ZEPEDA T A, FUENTES S, ECHAVARRÍA A. Effect of the divalent metal and the activation temperature of NiMoW and CoMoW on the dibenzothiophene hydrodesulfurization reaction[J]. Appl Catal B:Environ, 2014, 148-149:221-230. doi: 10.1016/j.apcatb.2013.10.057 [19] WANG C L, WU Z Z, TANG C Y, LI L H, WANG D Z. The effect of nickel content on the hydrodeoxygenation of 4-methylphenol over unsupported NiMoW sulfide catalysts[J]. Catal Commun, 2013, 32(0):76-80. https://www.sciencedirect.com/science/article/pii/S1566736712004578 [20] ZHANG H P, LIN H F, ZHENG Y, HU Y F, MACLENNAN A. The catalytic activity and chemical structure of nano MoS2 synthesized in a controlled environment[J]. React Chem Eng, 2016, 1(2):165-175. doi: 10.1039/C5RE00046G [21] CHEN G, WANG S P, YI R, TAN L F, LI H B, ZHOU M, YAN L T, JIANG Y B, TAN S, WANG D H, DENG S G, MENG X W, LUO H M. Facile synthesis of hierarchical MoS2-carbon microspheres as a robust anode for lithium ion batteries[J]. J Mater Chem A, 2016, 4(24):9653-9660. doi: 10.1039/C6TA03310E [22] YI Y J, ZHANG B S, JIN X, WANG L, WILLIAMS C T, XIONG G, SU D S, LIANG C H. Unsupported NiMoW sulfide catalysts for hydrodesulfurization of dibenzothiophene by thermal decomposition of thiosalts[J]. J Mol Catal A:Chem, 2011, 351(0):120-127. [23] LIU H, SU X, DUAN C Y, DONG X N, ZHU Z F. A novel hydrogen peroxide biosensor based on immobilized hemoglobin in 3D flower-like MoS2 microspheres structure[J]. Mater Lett, 2014, 122(0):182-185. https://www.sciencedirect.com/science/article/pii/S0925400516304233 [24] PANDEY K, YADAV P, MUKHOPADHYAY I. Electrochemical and electronic properties of flower-like MoS2 nanostructures in aqueous and ionic liquid media[J]. RSC Adv, 2015, 5(71):57943-57949. doi: 10.1039/C5RA09282E [25] WU K, WANG W Y, TAN S, ZHU G H, TAN L, YANG Y Q. Microwave-assisted hydrothermal synthesis of amorphous MoS2 catalysts and their activities in the hydrodeoxygenation of p-cresol[J]. RSC Adv, 2016, 6(84):80641-80648. doi: 10.1039/C6RA19007C [26] LIU Y, ZHOU X L, DING T, WANG C D, YANG Q. 3D architecture constructed via the confined growth of MoS2 nanosheets in nanoporous carbon derived from metal-organic frameworks for efficient hydrogen production[J]. Nanoscale, 2015, 7(43):18004-18009. doi: 10.1039/C5NR03810C [27] LAI W K, CHEN Z, ZHU J P, YANG L F, ZHENG J B, YI X D, FANG W P. A NiMoS flower-like structure with self-assembled nanosheets as high-performance hydrodesulfurization catalysts[J]. Nanoscale, 2016, 8(6):3823-3833. doi: 10.1039/C5NR08841K [28] WHIFFEN V M L, SMITH K J. Hydrodeoxygenation of 4-Methylphenol over Unsupported MoP, MoS2, and MoOx Catalysts[J]. Energy Fuels, 2010, 24(9):4728-4737. doi: 10.1021/ef901270h -





 下载:
下载: