Low temperature light-assisted hydrogen production from aqueous reforming ethylene glycol over Pt/Al2O3 and Pd/Al2O3 catalysts
-
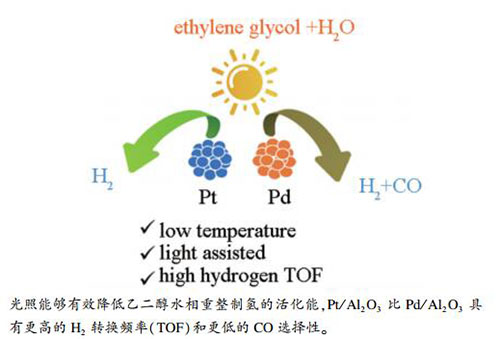
摘要: 采用浸渍还原法制备了氧化铝负载的Pt和Pd纳米颗粒催化剂,用于光辅助乙二醇水相重整制氢反应。结果表明,光照能够有效降低乙二醇水相重整制氢的活化能,Pt/Al2O3比Pd/Al2O3具有更高的H2转换频率(TOF)和更低的CO选择性。采用XRD、TEM、UV-vis等技术对催化剂的结构和形貌进行了表征,原位漫反射红外光谱(DRIFTS)表明,光照能促进乙二醇分子O-H键的断裂。理论计算表明,Pt/Al2O3催化乙二醇重整制氢反应产物中较低的CO选择性主要归因于CO在Pt表面较小的反应能垒,能够较快与H2O解离的O反应生成CO2。Abstract: Al2O3 supported Pt and Pd nanoparticle catalysts were prepared by impregnation-reduction method, and employed in the photocatalytic aqueous-phase reforming of ethylene glycol. Light illumination can decrease the reaction activation energy remarkably. Pt/Al2O3 exhibits much higher H2 turnover frequency (TOF) and lower CO selectivity than Pd/Al2O3 catalyst. Their morphology and structure were characterized by XRD, TEM, UV-vis techniques. In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) indicates light can promote the cleavage of O-H bonds of ethylene glycol molecule. DFT calculation suggests the lower CO selectivity over Pt/Al2O3 catalyst can be attributed to the low energy barriers of reaction in the step of CO+O→CO2.
-
Key words:
- hydrogen production /
- ethylene glycol /
- photocatalysis /
- aqueous-phase reforming /
- metal catalyst
-
图 1 生物醇水相重整制氢反应路径示意图[4]
Figure 1 Reaction pathways of H2 production by aqueous-phase reforming of biomass-derived alcohols
图 4 不同温度下光照对(a) Pt/Al2O3和(c) Pd/Al2O3光助催化乙二醇水相重整制氢催化性能的影响; 不同光强对(b) Pt/Al2O3和(d) Pd/Al2O3光助催化乙二醇水相重整制氢催化性能的影响
Figure 4 Effect of temperature on catalytic performance in ethylene glycol aqueous phase reforming over (a) Pt/Al2O3 and (c) Pd/Al2O3; effect of light intensity on catalytic performance in ethylene glycol aqueous phase reforming over (b) Pt/Al2O3 and (d) Pd/Al2O3 reaction condition: ethylene glycol 2mL, H2O 18mL, catalyst 50mg, 3h, the light intensity for (a) and (c) is 0.1W/cm2, the reaction temperature of (b) and (d) is 150℃
表 1 Pt/Al2O3和Pd/Al2O3催化光暗活性对比
Table 1 Catalytic performance of Pt/Al2O3 and Pd/Al2O3 with or without light
Entry Catalyst TOF /h-1 Selectivity of gas products s/% Selectivity of liquid products s/% H2 CO CH4 CO2 ethanol aldehyde 1 Pt/Al2O3 dark 197.9 67.0 0.1 0.4 32.5 72.1 27.9 2 Pt/Al2O3 light 1152.5 71.2 0.3 2.3 26.2 64.7 35.3 3a Pt/Al2O3 dark - - - - - - - 4a Pt/Al2O3 light - - - - - - - 5b - dark - - - - - - - 6b - light - - - - - - - 7 Pd/Al2O3 dark 11.0 66.7 13.8 0.6 18.9 95.3 4.7 8 Pd/Al2O3 light 120.5 65.4 24.4 3.5 6.7 89.6 10.4 9a Pd/Al2O3 dark - - - - - - - 10a Pd/Al2O3 light - - - - - - - reaction conditions: ethylene glycol 2mL, H2O 18mL, catalyst 50mg, 150℃, 3h, the light intensity for 0.1W/cm2 for light reaction; a: without ethylene glycol; b: without catalyst 表 2 Pt(111)和Pd(111)面上不同基元步骤上计算的反应能垒(Eb)和反应能(ΔE)
Table 2 Calculated energy barriers of reaction (Eb), reaction energies (ΔE) of the elementary steps on Pt(111), Pd(111) surfaces
Elementary step Pd(111) Pt(111) Eb/eV ΔE/eV Eb/eV ΔE/eV R1 H2O$ \to $H+OH 1.42 -0.02 2.07 0.73 R2 OH$ \to $H+O 1.34 0.15 1.29 0.06 R3 OH+OH$ \to $O+H2O 0.83 -0.42 0.58 -0.60 R4 H+H$ \to $H2 1.85 1.07 1.50 0.64 R5 CO+O$ \to $ CO2 1.97 -0.35 1.47 -0.91 -
[1] TURNER J A. Sustainable hydrogen production[J]. Science, 2004, 305(5686):972-974. doi: 10.1126/science.1103197 [2] KUEHNEL M F, REISNER E. Solar hydrogen generation from lignocellulose[J]. Angew Chem Int Ed, 2018, 57(13):3290-3296. doi: 10.1002/anie.201710133 [3] LINDSTROM B, PETTERSSON L J. Hydrogen generation by steam reforming of methanol over copper-based catalysts for fuel cell applications[J]. Int J Hydrogen Energy, 2001, 26(9):923-933. doi: 10.1016/S0360-3199(01)00034-9 [4] CORTRIGHT R D, DAVDA R R, DUMESIC J A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water[J]. Nature, 2002, 418(6901):964-967. doi: 10.1038/nature01009 [5] 刘洋, 朱善辉, 李俊汾, 秦张峰, 樊卫斌, 王建国.纳米片层二硫化钼负载PtCo双金属催化甲醇水相重整制氢[J].燃料化学学报, 2019, 47(7):799-805. doi: 10.3969/j.issn.0253-2409.2019.07.004LIU Yang, ZHU Shan-hui, LI Jun-fen, QIN Zhang-feng, FAN Wei-bin, WANG Jian-guo. Catalytic performance of bimetallic PtCo supported on nanosheets MoS2 in aqueous phase reforming of methanol to hydrogen[J]. J Fuel Chem Technol, 2019, 47(7):799-805. doi: 10.3969/j.issn.0253-2409.2019.07.004 [6] 张磊, 潘立卫, 倪长军, 赵生生, 王树东, 胡永康, 王安杰, 蒋凯.甲醇水蒸气重整制氢反应条件的优化[J].燃料化学学报, 2013, 41(1):116-122. doi: 10.3969/j.issn.0253-2409.2013.01.019ZHANG Lei, PAN Li-wei, NI Chang-jun, ZHAO Sheng-sheng, WANG Shu-dong, HU Yong-kang, WANG An-jie, JIANG Kai. Catalytic performance of bimetallic PtCo supported on nanosheets MoS2 in aqueous phase reforming of methanol to hydrogen[J]. J Fuel Chem Technol, 2013, 41(1):116-122. doi: 10.3969/j.issn.0253-2409.2013.01.019 [7] VADYA P D, LOPZE-SANCHEZ J A. Review of hydrogen production by catalytic aqueous-phase reforming[J]. Chemistryselect, 2017, 2(22):6563-6576. doi: 10.1002/slct.201700905 [8] CORONADO I, STEKROVA M, RENⅡKAINEN M, SIMELL P, LEFFERTS L, LEHTONEN J. A review of catalytic aqueous-phase reforming of oxygenated hydrocarbons derived from biorefinery water fractions[J]. Int J Hydrogen Energy, 2016, 41(26):11003-11032. doi: 10.1016/j.ijhydene.2016.05.032 [9] XIONG H, DELARIVA A, WANG Y, DATYE A. Low-temperature aqueous-phase reforming of ethanol on bimetallic PdZn catalysts[J]. Catal Sci Technol, 2015, 5(1):254-263. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=224e52a932a4b1e3f0902d6f80d8b846 [10] CORONADO I, STEKROVA M, MORENO L G, REINIKANINEN M, SIMELL P, KARINEN R, LEHTONEN J. Aqueous-phase reforming of methanol over nickel-based catalysts for hydrogen production[J]. Biomass Bioenergy, 2017, 106:29-37. doi: 10.1016/j.biombioe.2017.08.018 [11] HUBER G W, DUMESIC J A. An overview of aqueous-phase catalytic processes for production of hydrogen and alkanes in a biorefinery[J]. Catal Today, 2006, 111(1):119-132. doi: 10.1016-j.cattod.2005.10.010/ [12] SARINA S, ZHU H, XIAO Q, JAATINEN E, JIA J, HUANG Y, ZHENG Z, WU H. Viable photocatalysts under solar-spectrum irradiation:Nonplasmonic metal nanoparticles[J]. Angew Chem Int Ed, 2014, 53(11):2935-2940. doi: 10.1002/anie.201308145 [13] TANA T, GUO X, XIAO Q, HUANG Y, SARINA S, CHRISTOPHER P, JIA J, WU H, ZHU H. Non-plasmonic metal nanoparticles as visible light photocatalysts for the selective oxidation of aliphatic alcohols with molecular oxygen at near ambient conditions[J]. Chem Commun, 2016, 52(77):11567-11570. doi: 10.1039/C6CC05186C [14] GUO X, JIAO Z, JIN G, GUO X. Photocatalytic fischer-tropsch synthesis on graphene-supported worm-like ruthenium nanostructures[J]. ACS Catal, 2015, 5(6):3836-3840. doi: 10.1021/acscatal.5b00697 [15] LIU H, T. DAO D, LIU L, MENG X, NAGAO T, YE J. Light assisted CO2 reduction with methane over group VⅢ metals:Universality of metal localized surface plasmon resonance in reactant activation[J]. Appl Catal B:Environ, 2017, 209, 183-189. doi: 10.1016/j.apcatb.2017.02.080 [16] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1996, 77(18):3865-3868. doi: 10.1103/PhysRevLett.77.3865 [17] LIU H, WU Z, WANG R, DONG M, WANG G, QIN Z, Ma J, HUANG Y, WANG J, FAN W. Structural and electronic feature evolution of Au-Pd bimetallic catalysts supported on graphene and SiO2 in H2 and O2[J]. J Catal, 2019, 376:44-56. doi: 10.1016/j.jcat.2019.06.049 [18] HENKELMAN G, JONSSON H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points[J]. J Chem Phys, 2000, 113(22):9978-9985. doi: 10.1063/1.1323224 [19] HENKELMAN G, UBERUAGA B P, JONSSON H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. J Chem Phys, 2000, 113(22):9901-9904. doi: 10.1063/1.1329672 [20] ZHU T, LI J, YIP S. Atomistic study of dislocation loop emission from a crack tip[J]. Phys Rev Lett, 2004, 93(2):025503. doi: 10.1103/PhysRevLett.93.025503 [21] WANG Y, WANG G. A systematic theoretical study of water gas shift reaction on Cu(111) and Cu(110):Potassium effect[J]. ACS Catal, 2019, 9(3):2261-2274. doi: 10.1021/acscatal.8b04427 [22] LIU Z, UANG Y, XIAO Q, ZHU H. Selective reduction of nitroaromatics to azoxy compounds on supported Ag-Cu alloy nanoparticles through visible light irradiation[J]. Green Chem, 2016, 18(3):817-825. doi: 10.1039/C5GC01726B [23] XIAO Q, LIU Z, BO A, ZAVAHIR S, SARINA S, BOTTLE S, RICHES J D, ZHU H. Catalytic transformation of aliphatic alcohols to corresponding esters in O2 under neutral conditions using visible-light irradiation[J]. J Am Chem Soc, 2015, 137(5):1956-1966. doi: 10.1021/ja511619c [24] HAO C H, GUO X N, PAN Y T, CHEN S, JIAO Z F, YANG H, GUO X Y. Visible-light-driven selective photocatalytic hydrogenation of cinnamaldehyde over Au/SiC catalysts[J]. J Am Chem Soc, 2016, 138(30):9361-9364. doi: 10.1021/jacs.6b04175 [25] LINIC S, ASLAM U, BOERIGTER C, MORABITO M. Photochemical transformations on plasmonic metal nanoparticles[J]. Nat Mater, 2015, 14(6):567-576. doi: 10.1038/nmat4281 [26] LINIC S, CHRISTOPHER P, INGRAM D B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy[J]. Nat Mater, 2011, 10(12):911-921. doi: 10.1038/nmat3151 [27] LIN L, ZHOU W, GAO R, YAO S, ZHANG X, XU W, ZHENG S, JIANG Z, YU Q, LI Y, SHI C, WEN X, MA D. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544(7648):80-83. doi: 10.1038/nature21672 [28] OCHOA J V, TREVISANUT C, MILLET J M, BUSCA G, CAVANI F. In situ DRIFTS-MS study of the anaerobic oxidation of ethanol over spinel mixed oxides[J]. J Phy Chem C, 2013, 117(45):23908-23918. doi: 10.1021/jp409831t [29] CESAR D V, SANTORI G F, POMPEO F, BALDANZA M A, HENRIQUES C A, LOMBAEDO E, SCHMAL M, CORNAGLIA L, NICHIO N N. Hydrogen production from ethylene glycol reforming catalyzed by Ni and Ni-Pt hydrotalcite-derived catalysts[J]. Int J Hydrogen Energy, 2016, 41(47):22000-22008. doi: 10.1016/j.ijhydene.2016.07.168 -





 下载:
下载: