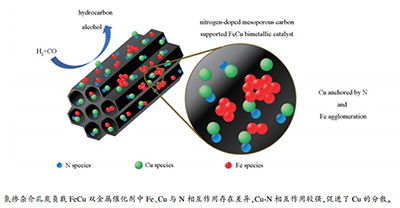
Nitrogen-doped mesoporous carbon supported FeCu bimetallic catalyst and its CO hydrogenation performance
-
摘要: 利用氮掺杂介孔炭负载FeCu双金属,改变Fe/Cu组成,考察催化剂结构性质特征及其CO加氢反应性能。结果表明,Fe、Cu与N相互作用存在差异,Cu-N相互作用较强,并直接促进了Cu的分散。在较高的金属负载量(45.0%-50.0%,质量分数)下,Cu仍保持了与N一致的均匀分布特征,催化剂表面Fe/Cu组成也因为Fe、Cu分布特征差异而小于体相,这与常见Fe-Cu体系明显不同。在所用预处理条件(300℃的H2气氛)下,Fe未被完全还原,H主要与Fe-O作用,以Fe-O-H形式存在,而Cu-N作用较强,金属Cu与H作用较弱,使得催化剂表面活性氢碳比降低,导致C5+选择性随Fe/Cu比值的减小逐渐增加。与此同时,载体向负载金属的电子偏移能力也随着Fe/Cu比值的减小逐渐增强,促使催化剂表面碱性随Cu含量的增加逐渐增强,最终导致C5+选择性、醇选择性进一步增加。Abstract: In this work, nitrogen-doped mesoporous carbon (NDMC) was prepared by a hard template method, and the NDMC supported FeCu bimetallic catalysts were prepared by an impregnation method. The physical and chemical properties and CO hydrogenation performance of the catalysts with varying Fe/Cu ratios were studied. The results indicated that Cu-N had strong interaction which directly promoted Cu dispersion on the support. At a relatively high metal loading (45.0%-50.0%), Cu maintained uniform distribution similar to that of N, and the ratios of Fe/Cu on the catalyst surface were smaller than those in the bulk phase, which were different from precipitated Fe-Cu bimetallic catalysts. The XPS results showed that Cu was an electron donor, and the electrons in the Cu-N shifted to Fe. Compared with Fe/NDMC, the reduction of FexCuy/NDMC was facilitated, and their CO hydrogenation activity was significantly increased. Under the pretreatment conditions (H2, 300℃), Fe was not completely reduced, and H might mainly interact with Fe-O in the form of Fe-O-H, while Cu-N interaction was stronger than Cu-H, resulting in a decrease in the ratio of surface active carbon/hydrogen, leading to a gradual increase in C5+ selectivity with the decrease of Fe/Cu ratio. Meanwhile, the introduction of Cu inhibited CO dissociation to some extent, and the electron migration ability of the support to the metal gradually increased with decreasing Fe/Cu ratio, and as a result the surface alkalinity of the catalysts increased with increasing Cu content, leading to further enhancement of C5+ selectivity and alcohol selectivity.
-
Key words:
- nitrogen-doped mesoporous carbon /
- FeCu bimetal /
- interaction /
- CO hydrogenation
-
表 1 氮掺杂介孔炭及其负载FeCu双金属催化剂的织构信息
Table 1 Textural parameters of NDMC and FeCu/NDMC
Sample Specific surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average pore size d/nm NDMC 769.4 0.799 6.5 Fe/NDMC 256.2 0.352 5.8 Fe8Cu2/NDMC 258.4 0.346 5.8 Fe6Cu4/NDMC 268.7 0.383 5.9 Fe5Cu5/NDMC 254.5 0.359 5.8 表 2 氮掺杂介孔炭及其负载FeCu双金属催化剂的元素含量
Table 2 Element contents of NDMC and FeCu/NDMC
Sample Metal loading w/%a Surface atom ratio/%b Fe/Cu (atomic ratio) Bulk atom ratio of Fe/Cua N C O Fe Cu NDMC - 20.53 73.21 6.26 - - - - Fe8Cu2/NDMC 45.92 12.45 52.37 23.46 7.59 4.13 1.84 3.83 Fe6Cu4/NDMC 46.28 13.28 52.63 23.16 5.80 5.13 1.13 1.45 Fe5Cu5/NDMC 46.39 11.65 51.05 26.50 5.26 5.54 0.95 0.97 a: obtained by ICP measurement;
b: obtained by XPS measurement表 3 氮掺杂介孔炭及其负载的FeCu双金属催化剂的组成
Table 3 Surface composition of NDMC and FeCu/NDMC
Catalyst Binding energy E/eV CuA2+/ (CuA2++CuB2+)a pyridinic N pyrrolic N graphitic N Fe 2p3/2 Fe 2p1/2 Cu 2p3/2 Cu 2p1/2 NDMC 398.3 400.1 402.1 - - - - - Fe8Cu2/NDMC 398.5 400.3 402.2 710.4 724.3 933.6 952.5 0.63 Fe6Cu4/NDMC 398.9 400.5 402.2 710.4 723.7 933.9 953.7 0.71 Fe5Cu5/NDMC 399.2 400.7 402.1 710.6 723.5 934.4 954.0 0.69 a: area ratio of CuB2+/(CuA2++CuB2+) 表 4 不同Fe/Cu组成的FexCuy/NDMC催化剂的反应性能
Table 4 Catalytic performance of FexCuy/NDMC in CO hydrogenation
Catalyst Temperature
t/℃CO conversion
x/%Selectivity in hydrocarbon smol/% Selectivity sC/% CH4 C2-4 C5+ HCs ROH CO2 Fe8Cu2/NDMC 240 22.8 19.1 45.3 35.7 78.8 9.7 11.5 250 37.7 18.3 51.6 30.0 77.0 9.4 13.7 Fe6Cu4/NDMC 240 19.4 20.5 24.8 54.7 75.3 12.0 12.7 250 32.8 19.4 30.5 50.1 71.9 10.8 17.3 Fe5Cu5/NDMC 240 18.7 19.3 22.5 58.2 75.4 13.9 10.7 250 25.2 20.4 26.2 53.4 74.4 10.8 14.8 reaction conditions: p=3.0 MPa, H2/CO=2, GHSV=3000 mL/(gcat·h), the values were obtained at the steady-state after 48 h on stream -
[1] LIAND C D, LI Z J, DAI S. Mesoporous carbon materials:synthesis and modification[J]. Angew Chem Int Ed Eng, 2008, 47(20):3696-3717. doi: 10.1002/(ISSN)1521-3773 [2] FU T J, LI Z H. Review of recent development in Co-based catalysts supported on carbon materials for Fischer-Tropsch synthesis[J]. Chem Eng Sci, 2015, 135:3-20. doi: 10.1016/j.ces.2015.03.007 [3] XIONG H F, JEWELL L L, COVILLE N J. Shaped carbons as supports for the catalytic conversion of syngas to clean fuels[J]. ACS Catal, 2015, 5(4):2640-2658. doi: 10.1021/acscatal.5b00090 [4] 刘云朋, 周洁, 李中坚, 雷乐成.氮掺杂有序介孔碳的研究进展[J].现代化工, 2014, 34(6):19-22. http://d.old.wanfangdata.com.cn/Periodical/xdhg201406006LIU Yun-peng, ZHOU Jie, LI Zhong-jian, LEI Le-cheng. Research progress of N-doped ordered mesoporous carbon[J]. Mod Chem Ind, 2014, 34(6):19-22. http://d.old.wanfangdata.com.cn/Periodical/xdhg201406006 [5] 李烁, 姚楠.掺氮碳基材料在费托合成反应中的应用[J].化工进展, 2015, 34(11):3933-3937. http://d.old.wanfangdata.com.cn/Periodical/hgjz201511020LI Suo, YAO Nan. Application of nitrogen-doped carbon materials in Fischer-Tropsch synthesis reaction[J]. Chem Ind Eng Prog, 2015, 34(11):3933-3937. http://d.old.wanfangdata.com.cn/Periodical/hgjz201511020 [6] SANKAR M, DIMITRATOS N, MIEDZIAK P J, WELLS P P, KIELY C J, HUTCHING G J. Designing bimetallic catalysts for a green and sustainable future[J]. Chem Soc Rev, 2012, 41(24):8099-8139. doi: 10.1039/c2cs35296f [7] WANG G H, CAO Z W, GU D, PFANDER N, SWERTZ A C, SPLIETHOFF B, BONGARD H J, WEIDENTHALER C, SCHMIDT W, RINALDI R, SCHUTH F. Nitrogen-doped ordered mesoporous carbon supported bimetallic PtCo nanoparticles for upgrading of biophenolics[J]. Angew Chem Int Ed, 2016, 55(31):8850-8855. doi: 10.1002/anie.201511558 [8] XIAO K, QI X Z, BAO Z H, WANG X X, ZHONG L S, FANG K G, LIN M G, SUN Y H. CuFe, CuCo and CuNi nanoparticles as catalysts for higher alcohol synthesis from syngas:A comparative study[J]. Catal Sci Technol, 2013, 3(6):1591-1602. doi: 10.1039/c3cy00063j [9] XIAO K, BAO Z H, QI X Z, WANG X X, ZHONG L S, LIN M G, FANG K G. Unsupported CuFe bimetallic nanoparticles for higher alcohol synthesis via syngas[J]. Catal Commun, 2013, 40:154-157. doi: 10.1016/j.catcom.2013.06.024 [10] KHODAKOV A Y, CHU W, FONGARLAND P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels[J]. Chem Rev, 2007, 107(5):1692-1744. doi: 10.1021/cr050972v [11] LI S Z, LI A W, KRISHNAMOORTHY S, IGLESIA E. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts[J]. Catal Lett, 2001, 77(4):197-205. doi: 10.1023/A:1013284217689 [12] GAO W, ZHAO Y F, LIU J M, HUANG Q W, HE S, LI C M, ZHAO J W, WEI M. Catalytic conversion of syngas to mixed alcohols over CuFe-based catalysts derived from layered double hydroxides[J]. Catal Sci Technol, 2013, 3(5):1324-1332. doi: 10.1039/c3cy00025g [13] XIAO K, BAO Z H, QI X Z, WANG X X, ZHONG L S, FANG K G, LIN M G, SUN Y H. Structural evolution of CuFe bimetallic nanoparticles for higher alcohol synthesis[J]. J Mol Catal A:Chem, 2013, 378(11):319-325. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4dd29ff2a1b453d66d1921ffbf802631 [14] SHI X P, YU H B, GAO S, LI X Y, FANG H H, LI R J, LI Y Y, ZHANG L J, LIANG X L, YUAN Y Z. Synergistic effect of nitrogen-doped carbon-nanotube-supported Cu-Fe catalyst for the synthesis of higher alcohols from syngas[J]. Fuel, 2017, 210:241-248. doi: 10.1016/j.fuel.2017.08.064 [15] KIATPHUENGPORN S, CHAREONPANICH M, LIMTRAKUL J. Effect of unimodal and bimodal MCM-41 mesoporous silica supports on activity of Fe-Cu catalysts for CO2 hydrogenation[J]. Chem Eng J, 2014, 240:527-533. doi: 10.1016/j.cej.2013.10.090 [16] WANG S, ZHAO Q, WEI H, WANG J Q, CHO M, CHO H S, TERASAKI O, WAN Y. Aggregation-free gold nanoparticles in ordered mesoporous carbons:Toward highly active and stable heterogeneous catalysts[J]. J Am Chem Soc, 2013, 135(32):11849-11860. doi: 10.1021/ja403822d [17] LIU G G, CHEN Q J, OYUNKHAND E, DING S Y, YAMANE N, YANG G H, YONEYAMA Y, TSUBAKI N. Nitrogen-rich mesoporous carbon supported iron catalyst with superior activity for Fischer-Tropsch synthesis[J]. Carbon, 2018, 130:304-314. doi: 10.1016/j.carbon.2018.01.015 [18] XIONG H F, MOYO M, RAYNER M, JEWELL L L, BILLING D G, COVILLE N. Autoreduction and catalytic performance of a cobalt Fischer-Tropsch synthesis catalyst supported on nitrogen-doped carbon spheres[J]. ChemCatChem, 2010, 2(5):514-518. doi: 10.1002/cctc.200900309 [19] DING Y Q, YANG J H, YANG G Z, LI P. Fabrication of ordered mesoporous carbons anchored with MnO nanoparticles through dual-templating approach for supercapacitors[J]. Ceram Int, 2015, 41(8):9980-9987. doi: 10.1016/j.ceramint.2015.04.078 [20] LU J Z, YANG L J, XU B L, WU Q, ZHANG D, YUAN S J, ZHAI Y P, WANG X Z, FAN Y N, HU Z. Promotion effects of nitrogen doping into carbon nanotubes on supported iron Fischer-Tropsch catalysts for lower olefins[J]. ACS Catal, 2014, 4(2):613-621. doi: 10.1021/cs400931z [21] CHE R C, LIANG C Y, SHI H L, ZHOU X G, YANG X N. Electron energy-loss spectroscopy characterization and microwave absorption of iron-filled carbon-nitrogen nanotubes[J]. Nanotechnol, 2007, 18(35):355705. doi: 10.1088/0957-4484/18/35/355705 [22] KIM S J, PARK Y J, RA E J, KIM K K, AN K H, LEE Y H, CHOI J Y, PARK C H, DOO S G, PARK M H, YANG C W. Defect-induced loading of Pt nanoparticles on carbon nanotubes[J]. Appl Phy Lett, 2007, 90(2):169. http://cn.bing.com/academic/profile?id=d27ae3c591d5f6db681c7e7d91661e15&encoded=0&v=paper_preview&mkt=zh-cn [23] BARTOLOME L, IMRAN M, LEE K G, SANGALANG A, AHN J K, KIM D H. Superparamagnetic γ-Fe2O3 nanoparticles as an easily recoverable catalyst for the chemical recycling of PET[J]. Green Chem, 2014, 16(1):279-286. doi: 10.1039/C3GC41834K [24] LI F, ZHANG L H, EVANS D G, DUAN X. Structure and surface chemistry of manganese-doped copper-based mixed metal oxides derived from layered double hydroxides[J]. Colloids Surfaces A, 2004, 244(1):169-177. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7ee0110808fbd57b633b0f8aa7605439 [25] LUK H T, MONDELLI C, MITCHELL S, SIOL S, STEWART J A, FERRE D C, PEREZ-RAMIREZ J. Role of carbonaceous supports and potassium promoter on higher alcohols synthesis over copper-iron catalysts[J]. ACS Catal, 2018, 8(10):9604-9618. doi: 10.1021/acscatal.8b02714 [26] SUN F G, LIU J, CHEN H C, ZHANG Z X, QIAO W M, LONG D H, LING L C. Nitrogen-rich mesoporous carbons:Highly efficient, regenerable metal-free catalysts for low-temperature oxidation of H2S[J]. ACS Catal, 2013, 3(5):862-870. doi: 10.1021/cs300791j [27] BAGUS P S, ILTON E, NELIN C J. The interpretation of XPS spectra:Insights into materials properties[J]. Surf Sci Rep, 2013, 68(2):273-304. doi: 10.1016/j.surfrep.2013.03.001 [28] YANG X M, WEI Y, SU Y L, ZHOU L P. Characterization of fused Fe-Cu based catalyst for higher alcohols synthesis and DRIFTS investigation of TPSR[J]. Fuel Process Technol, 2010, 91(9):1168-1173. doi: 10.1016/j.fuproc.2010.03.032 [29] ZHANG C H, ZHAO G Y, LIU K K, YANG Y, XIANG H W, LI Y W. Adsorption and reaction of CO and hydrogen on iron-based Fischer-Tropsch synthesis catalysts[J]. J Mol Catal A:Chem, 2010, 328(1/2):35-43. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ffab8b7babce08c8ff4afb536239815a [30] BLIEM R, VAN DER HOEVEN J, ZAVODNY A, GAMBA O, PAVELEC J, DE JONGH P E, SCHMID M, DIEBOLD U, PARKINSON G S. An atomic-scale view of CO and H2 oxidation on a Pt/Fe3O4model catalyst[J]. Angew Chem Int Ed, 2015, 127(47):14205-14208. doi: 10.1002/ange.201507368 [31] AL-DOSSARY M, FIERRO J L G, SPIVEY J J. Cu-promoted Fe2O3/MgO-based Fischer-Tropsch catalysts of biomass-derived syngas[J]. Ind Eng Chem Res, 2015, 54(3):911-921. doi: 10.1021/ie504473a [32] MAO W Y, MA H F, ZHANG H T, SUN Q W, YING W Y. Influence of copper loading on the surface species and catalytic properties in the formation of oxygenated by-products during FTS over FeCuKLa/SiO2catalysts[J]. Catal Lett, 2012, 142(9):1098-1106. doi: 10.1007/s10562-012-0865-6 [33] KANG S H, KOO H M, KIM A R, LEE D H, RYU J H, YOO Y D, BAE J W. Correlation of the amount of carbonaceous species with catalytic performance on iron-based Fischer-Tropsch catalysts[J]. Fuel Process Technol, 2013, 109:141-149. doi: 10.1016/j.fuproc.2012.09.052 [34] ZHANG C H, YANG Y, TENG B T, LI T Z, ZHENG H Y, XIANG H W, LI Y W. Study of an iron-manganese Fischer-Tropsch synthesis catalyst promoted with copper[J]. J Catal, 2006, 237(2):405-415. http://cn.bing.com/academic/profile?id=c65080a18786083e192f4d736240d190&encoded=0&v=paper_preview&mkt=zh-cn [35] CHONCO Z H, LODYA L, CLAEYS M, STEEN E V. Copper ferrites:A model for investigating the role of copper in the dynamic iron-based Fischer-Tropsch catalyst[J]. J Catal, 2013, 308:363-373. doi: 10.1016/j.jcat.2013.08.012 -





 下载:
下载: