Experimental study on selective catalytic reduction of NO by C3H6 over Fe-Ag/Al2O3 catalysts
-
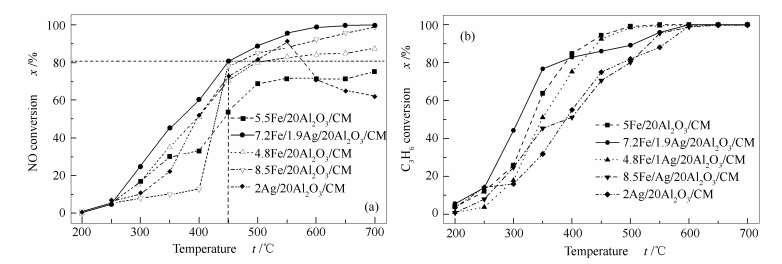
摘要: 以蜂窝状陶瓷为载体,采用溶胶凝胶法和浸渍法制备了不同Fe/Ag负载量的Fe-Ag/Al2O3催化剂。以C3H6为还原剂,在模拟烟气条件下和200-700 ℃范围内,程序控温的陶瓷管流动反应器上进行了催化还原NO的性能评估。结果表明,7.2Fe/1.9Ag/20Al2O3/CM在500和550 ℃时催化C3H6还原NO的脱硝效率分别超过90%和达到100%。铁离子能有效地提高Ag/20Al2O3/CM催化剂抵抗烟气中的SO2和H2O的能力。结果表明,当烟气中含有体积分数为0.02%的SO2和8%的H2O时,在500 ℃时7.2Fe/1.9Ag/20Al2O3/CM催化C3H6还原NO的脱硝效率不受影响,在6 h的连续实验中保持90%的脱硝效率而没有下降。而未经铁离子修饰的2Ag/20Al2O3/CM的催化活性则受烟气中的SO2和H2O影响很大,0.02%的SO2和8%的H2O分别使2Ag/20Al2O3/CM在500 ℃时催化C3H6还原NO的脱硝效率迅速从70%分别下降至46%和25%。XRD和SEM表征结果表明,经铁离子修饰后的7.2Fe/1.9Ag/20Al2O3/CM催化剂中,形成了AgFeO2以及Fe3+,催化剂表面变得疏松多孔,形成以Fe3O4为主的针状和片状晶体。H2-TPR结果表明,7.2Fe/1.9Ag/20Al2O3/CM比Ag/20Al2O3/CM在更宽的温度范围内具有更好的还原特性。吡啶吸附红外光谱(Py-FTIR)实验结果显示,Fe增加了催化剂表面的Lewis酸性位。
-
关键词:
- 选择性催化脱硝 /
- 丙烯 /
- Fe-Ag/Al2O3催化剂
Abstract: Sol-gel and impregnation methods were used to prepare the Fe/Ag/Al2O3 catalysts supported on the monolithic cordierite with different Fe/Ag loading ratios. The catalytic performance to reduce NO with C3H6 was evaluated in a one-dimensional electrically heated temperature programmed ceramic tubular reactor in simulated flue gas atmosphere at 200-700 ℃. The results show that the NO reduction efficiency on 7.2Fe/1.9Ag/20Al2O3/CM with C3H6 is more than 90% and reaches about 100% at the temperatures of 500 ℃ and 550 ℃ respectively. Iron can effectively improve the ability of Ag/20Al2O3/CM catalysts to resist SO2 and H2O in flue gas. When SO2 and H2O are 0.02% and 8% in the flue gas, the NO reduction efficiency is almost not influenced on 7.2Fe/1.9Ag/20Al2O3/CM at 500 ℃. The 90% NO reduction efficiency is maintained during 6 h without decrease. However, the catalytic activity of 2Ag/20Al2O3/CM without iron modification is strongly influenced by SO2 and H2O in the flue gas. The NO reduction efficiency on Ag/20Al2O3/CM decreases rapidly from about 70% to 46% and 25% respectively, when the SO2 and H2O are 0.02% and 8% in the flue gas. The results of XRD and SEM of the catalyst show that AgFeO2 and Fe3+ are formed in the 7.2Fe/1.9Ag/20Al2O3/CM catalyst after the modification by iron, and the surface of the catalyst become loose and porous, forming Fe3O4-based needle-like and flaky crystals. H2-TPR results show that 7.2Fe/1.9Ag/20Al2O3/CM has better reduction properties than Ag/20Al2O3/CM in the wider temperature range. Pyridine adsorption Infrared Spectroscopy (Py-FTIR) experimental results show that Fe increases the Lewis acid sites in the catalyst surface.-
Key words:
- selective catalytic reduction of NO /
- C3H6 /
- Fe-Ag/Al2O3 catalyst
-
图 4 不同温度时SO2和H2O对7.2Fe/1.9Ag/20Al2O3/CM和2Ag/20Al2O3/CM催化C3H6还原NO的影响
Figure 4 Effect of SO2 and H2O on C3H6-SCR of NO by 7.2Fe/1.9Ag/20Al2O3/CM(a) and 2Ag/20Al2O3/CM(b) at different temperature
—○—: 7.2Fe/1.9Ag/20Al2O3/CM, with SO2 and H2O; —●—: 7.2Fe/1.9Ag/20Al2O3/CM, without SO2 and H2O; --■--: 2Ag/20Al2O3/CM, without SO2 and H2O; --□--: 2Ag/20Al2O3/CM, with SO2 and H2O;
表 1 不同负载量样品的微孔隙特性
Table 1 Textural properties of different Fe/Ag loading on Al2O3
Catalyst ABET/ (m2·g-1) νp/ (cm3·g-1) dp/ nm 20Al2O3/CM[16] 27 0.076 4.4 2Ag/20Al2O3/CM 9 0.042 17.7 7.2Fe/1.9Ag/20Al2O3/CM 23 0.054 9.23 5.5Fe/20Al2O3/CM 19 0.039 6.54 表 2 三组样品中酸性位的含量
Table 2 Acid content of different Fe/Ag loading on Al2O3
Sample 150 ℃ 300 ℃ B /(mmol·g-1) L /(mmol·g-1) B /(mmol·g-1) L /(mmol·g-1) 20Al2O3/CM 0 0.001 20 0 0.000 60 2Ag/20Al2O3/CM 0 0.001 58 0 0.000 63 7.2Fe/1.9Ag/20Al2O3/CM 0 0.002 33 0 0.001 33 -
[1] HELD W, KÖNIG A, RICHTER T, PUPPE L. Catalytic NOx reduction in net oxidizing exhaust gas[J]. SAE Trans, 1990, 99(4):209-216. https://www.researchgate.net/publication/285442659_Catalytic_NOx_Reduction_in_Net_Oxidizing_Exhaust_Gas [2] MIYADERA T, YOSHIDA K. Alumina-supported catalysts for the selective reduction of nitric oxide by propene[J]. Chem Lett, 1993, 2(9):1483-1486. http://ci.nii.ac.jp/naid/130004419163 [3] WANG J, HE H, FENG Q, YU Y, YOSHIDA K. Selective catalytic reduction of NOx with C3H6 over an Ag/Al2O3 catalyst with a small quantity of noble metal[J]. Catal Today, 2004, 93-95:783-789. doi: 10.1016/j.cattod.2004.06.071 [4] CHAIEB T, DELANNOY L, LOUIS C, THOMAS C. On the origin of the optimum loading of Ag on Al2O3 in the C3H6-SCR of NOx[J]. Appl Catal B:Environ, 2013, 142-143:780-784. doi: 10.1016/j.apcatb.2013.06.010 [5] THOMAS C. On an additional promoting role of hydrogen in the H2-assisted C3H6-SCR of NOx on Ag/Al2O3:A lowering of the temperature of formation-decomposition of the organo-NOx intermediates[J]. Appl Catal B:Environ, 2015, 162:454-462. doi: 10.1016/j.apcatb.2014.07.021 [6] KIM P S, KIM M K, CHO B K, NAM I S, OH S H. Effect of H2 on deNOx performance of HC-SCR over Ag/Al2O3:Morphological, chemical, and kinetic changes[J]. J Catal, 2013, 301(5):65-76. http://www.sciencedirect.com/science/article/pii/S0021951713000389 [7] XIE S, WANG J, HE H. Poisoning effect of sulphate on the selective catalytic reduction of NOx by C3H6 over Ag-Pd/Al2O3[J]. J Mol Catal A:Chem, 2007, 266(1/2):166-172. http://www.sciencedirect.com/science/article/pii/S1381116906013434 [8] BURCH R, WATLING T C. The effect of promoters on Pt/Al2O3 catalysts for the reduction of NO by C3H6 under lean-burn conditions[J]. Appl Catal B:Environ, 1997, 11(2):207-216. doi: 10.1016/S0926-3373(96)00043-4 [9] SALEM I, COURTOIS X, CORBOS E C, MARECOT P, DUPREZ D. NO conversion in presence of O2, H2O and SO2:Improvement of a Pt/Al2O3 catalyst by Zr and Sn, and influence of the reducer C3H6 or C3H8[J]. Catal Commun, 2008, 9(5):664-669. doi: 10.1016/j.catcom.2007.07.034 [10] GOULA M A, CHARISIOU N D, PAPAGERIDIS K N, DELIMITIS A, PAPISTA E, PACKATOURIDOU E, ILIOPOULOU E F, MARNELLOS G, KONSOLAKIS M, YENTEKIS I V. A comparative study of the H2-assisted selective catalytic reduction of nitric oxide by propene over noble metal (Pt, Pd, Ir)/γ-Al2O3 catalysts[J]. J Environ Chem Eng, 2016, 4(2):1629-1641. doi: 10.1016/j.jece.2016.02.025 [11] MORE P M, NGUYEN D L, DONARE M K, UMBARKAR S B, GRANGER P, DUJARDIN C. Activation by pretreatment of Ag-Au/Al2O3 bimetallic catalyst to improve low temperature HC-SCR of NOx for lean burn engine exhaust[J]. Appl Catal B:Environ, 2015, 174:145-156. http://www.sciencedirect.com/science/article/pii/S0926337315001071 [12] MORE P M, NGUYEN D L, DONGARE M K, UMBARKAR S B, NUNS N, GIRARDON J S, DUJARDIN C, LANCELOT C, MAMEDE A S, GRANGER P. Rational preparation of Ag and Au bimetallic catalysts for the hydrocarbon-SCR of NOx:Sequential deposition vs. coprecipitation method[J]. Appl Catal B:Environ, 2015, 162:11-20. doi: 10.1016/j.apcatb.2014.06.031 [13] MORE P M, JAGTAP N, KULAL A B, DONGARE M K, UMBARKER S B. Magnesia doped Ag/Al2O3-Sulfur tolerant catalyst for low temperature HC-SCR of NOx[J]. Appl Catal B:Environ, 2014, 144:408-415. doi: 10.1016/j.apcatb.2013.07.044 [14] 周皞, 廖文裕, 苏亚欣, 林辛越. H2O和SO2对甲烷在金属铁表面还原NO的影响[J].洁净煤技术, 2015, 21(2):51-55. http://d.wanfangdata.com.cn/Periodical/jjmjs201502011ZHOU Hao, LIAO Wen-yu, SU Ya-xin, LIN Xin-yue. Influence of H2O and SO2 on NO reduction by methane on the surface of iron[J].Clean Coal Technol, 2015, 21(2):51-55. http://d.wanfangdata.com.cn/Periodical/jjmjs201502011 [15] ZHOU H, SU Y X, LIAO W Y, ZHONG F C. NO reduction by propane over monolithic cordierite-based Fe/Al2O3 catalyst:Reaction mechanism and effect of H2O/SO2[J]. Fuel, 2016, 182:352-360. doi: 10.1016/j.fuel.2016.05.116 [16] ZHOU H, SU Y X, LIAO W Y, ZHONG F C. Preparation, characterization, and properties of monolithic Fe/Al2O3/cordierite catalysts for NO reduction with C2H6[J]. Appl Catal A:Gen, 2015, 505:402-409. doi: 10.1016/j.apcata.2015.08.025 [17] KYRⅡENKO P, POPOVYCH N, SOLOVIEV S, ORLYK S, DZWIGAJ S. Remarkable Activity of Ag/Al2O3/cordierite catalysts in SCR of NO with ethanol and butanol[J]. Appl Catal B:Environ, 2013, 140/141(2):691-699. http://www.sciencedirect.com/science/article/pii/S0926337313002841 [18] 苏亚欣, 任立铭, 苏阿龙, 邓文义.甲烷在金属铁及氧化铁表面还原NO的研究[J].燃料化学学报, 2013, 41(11):1393-1400. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18303.shtmlSU Ya-xin, REN Li-ming, SU A-long, DENG Wen-yi. NO reduction by methane on the surface of iron oxides[J]. J Fuel Chem Technol, 2013, 41(11):1393-1400. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18303.shtml [19] 苏亚欣, 陆哲惺, 周皞, 窦逸峰, 邓文义.丙烷在金属铁表面还原NO的实验研究[J].燃料化学学报, 2014, 42(12):1470-1477. doi: 10.3969/j.issn.0253-2409.2014.12.009SU Ya-xin, LU Zhe-xing, ZHOU Hao, DOU Yi-feng, DENG Wen-yi. Experimental study on NO reduction by propane over iron[J]. J Fuel Chem Technol, 2014, 42(12):1470-1477. doi: 10.3969/j.issn.0253-2409.2014.12.009 [20] 窦逸峰, 苏亚欣, 陆哲惺, 周皞, 邓文义.乙烷在金属铁表面还原NO的实验研究[J].燃料化学学报, 2015, 42(10):1273-1280. doi: 10.3969/j.issn.0253-2409.2015.10.017DOU Yi-feng, SU Ya-xin, LU Zhe-xing, ZHOU Hao, DENG Wen-yi. Experimental study of NO reduction by ethane over iron[J]. J Fuel Chem Technol, 2015, 42(10):1273-1280. doi: 10.3969/j.issn.0253-2409.2015.10.017 [21] ZHOU H, LI K K, ZHAO B T, DENG W Y, SU Y X, ZHONG F C. Surface properties and reactivity of Fe/Al2O3/cordierite catalysts for NO reduction by C2H6:Effects of calcination temperature[J]. Chem Eng J, 2017, 326:737-744. doi: 10.1016/j.cej.2017.06.018 [22] PUJILAKSONO B, JONSSON T, HALVARSSON M, SVENSSON J E, JOHANSSON L G. Oxidation of iron at 400-600℃ in dry and wet O[J]. Corros Sci, 2010, 52(5):1560-1569. doi: 10.1016/j.corsci.2010.01.002 [23] SU J H, LIU Q Y, LIU Z Y, HUANG Z G. Honeycomb CuO/Al2O3/cordierite catalyst for selective catalytic reduction of NO by NH3-Effect of Al2O3 coating[J]. Ind Eng Chem Res, 2008, 47(13):4295-4301. doi: 10.1021/ie800105p [24] KYRⅡENKO P, POPOVYCH N, SOLOVIEV S, ORLYK S, DZWIGAJ S. Remarkable Activity of Ag/Al2O3/cordierite catalysts in SCR of NO with ethanol and butanol[J]. Appl Catal B:Environ, 2013, 140-141(2):691-699. http://www.sciencedirect.com/science/article/pii/S0926337313002841 [25] KUL'KO E V, IVANOVA A S, LITVAK G S, KRYUKOVA G N, TSYBULYA S V. Preparation and microstruc-tural and textural characterization of single-phase aluminum oxides[J]. Kinet Catal, 2004, 45(5):714-721. doi: 10.1023/B:KICA.0000044984.09163.80 [26] KOUOTOU P M, TIAN Z Y, VIEKER H, BEYER A, GÖLZHÄUSER A, KOHSEHÖINGHAUS K. Selective Synthesis ofα-Fe2O3 thin films and effect of the deposition temperature and lattice oxygen on the catalytic combustion of propene[J]. J Mater Chem A, 2013, 1(35):10495-10504. doi: 10.1039/c3ta11354j [27] GONZÁLEZ-VELASCO J R, FERRET R, LÓPEZ-FONSECA R, GUTIÉRREZ-ORTIZ M A. Influence of particle size distribution of precursor oxides on the synthesis of cordierite by solid-state reaction[J]. Powder Technol, 2005, 153(1):34-42. doi: 10.1016/j.powtec.2005.01.022 [28] POPOVICH N A, KIRⅡENKO P I, SOLOV'EV S A, ORLIK S N, DZWIGAJ S. Role of active components of an Ag/Al2O3/cordierite catalyst in selective reduction of NO by ethanol[J]. Theor Exp Chem, 2012, 48(4):258-264. doi: 10.1007/s11237-012-9270-x [29] SATOSHI S, MIKA S, MASATAKA F. Enhancing effect of water vapor on the reduction of lean NOx by ethanol over an Ag/Al2O3 catalyst supported on cordierite honeycomb[J]. React Kinet Catal Lett, 1998, 64(2):239-246. doi: 10.1007/BF02475340 [30] ZHANG R, KALIAGUINE S. Lean reduction of NO by C3H6 over Ag/alumina derived from Al2O3, AlOOH and Al(OH)3[J]. Appl Catal B:Environ, 2008, 78(3/4):275-287. http://www.sciencedirect.com/science/article/pii/S0926337307002809 [31] RIEDEL T, CLAEYS M, SCHULZ H, SCHAUB G, NAM S S, JUN K W, CHOI M J, KISHAN G, LEE K W. Comparative study of Fischer-Tropsch synthesis with H2/CO and H2/CO2 syngas using Fe-and Co-based catalysts[J]. Appl Catal A:Gen, 1999, 186(1/2):201-213. http://www.sciencedirect.com/science/article/pii/S0926860X99001738 [32] LI J H, ZHU Y Q, RUI K, HAO J M. Improvement of catalytic activity and sulfur-resistance of Ag/TiO2-Al2O3 for NO reduction with propene under lean burn conditions[J]. Appl Catal B:Environ, 2008, 80(3):202-213. http://www.sciencedirect.com/science/article/pii/S0926337307002706 [33] WILLIAMS M F, FONFÉ B, SIEVERS C. Hydrogenation of tetralin on silica-alumina-supported Pt catalysts Ⅰ. Physicochemical characterization of the catalytic materials[J]. J Catal, 2012, 251(2):485-496. http://www.sciencedirect.com/science/article/pii/S0021951707002370 -





 下载:
下载: