Catalytic performance of SAPO-11/HZSM-5 composite supported Cr2O3 in the transformation of LPG to light olefins
-
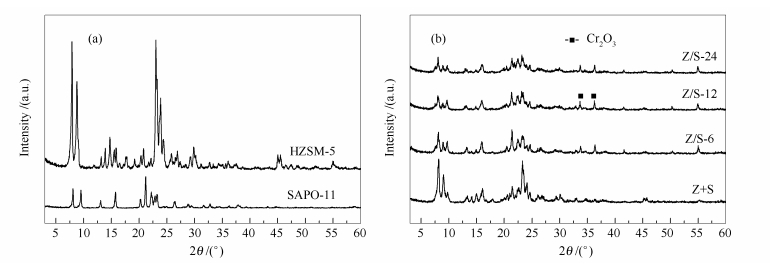
摘要: SAPO-11晶化液中预置HZSM-5合成了HZSM-5(核)/SAPO-11(壳)复合分子筛。以复合分子筛为载体负载10% Cr2O3,研究了其孔分布、酸性质及其对液化石油气(LPG)转化制乙烯和丙烯反应的催化性能。结果表明,复合分子筛由HZSM-5表面包覆不同厚度的SAPO-11微晶组成,随着晶化时间延长,复合分子筛壳层厚度增加。复合分子筛负载Cr2O3催化剂的介孔率先增加后降低;弱酸量先增加后降低,强酸强度增加,强酸量先降低后增加,强酸密度减小。复合分子筛载体在LPG选择转化反应中催化性能优于单个分子筛和机械混合分子筛,其中,晶化12 h合成样品负载Cr2O3用于LPG转化反应对原料总转化率和乙烯+丙烯选择性最高,分别为42.63%和65.89%,CH4和C5+选择性分别为6.32%和15.48%。通过控制晶化时间可调变壳层厚度、复合分子筛介孔率以及酸性质,改善产物分布。Abstract: HZSM-5(core)/SAPO-11(shell) composite molecular sieves were hydrothermally synthesized by crystallization of SAPO-11 outside HZSM-5 surface. After loading 10% Cr2O3, the pore distribution, acidity, and catalytic activity of the composite supported Cr2O3 in the transformation of liquefied petroleum gas (LPG) to ethene and propene were investigated. The results showed the HZSM-5 surface was coated with SAPO-11 microcrystalline of different thicknesses. With the increase of crystallization time, the shell thickness of the composite molecular sieves is increased; the mesoporosity and acidity can then be regulated by controlling the shell thickness. The composite supported Cr2O3 exhibits superior activity in the transformation of LPG to olefins than the single molecular sieves or mechanically mixed ones. The Cr2O3 catalyst supported on the composite by crystallization for 12 h gives the highest activity and selectivity to the target products; the feed conversion and the selectivity to ethene and propene are 42.63% and 65.89%, respectively, while the selectivities to CH4 and C5+ are 6.32% and 15.48%, respectively.
-
Key words:
- composite zeolite /
- chromium oxide /
- LPG /
- selective conversion /
- ethene and propene
-
表 1 分子筛负载Cr2O3样品的孔参数
Table 1 Pore parameters of the molecular sieve supported Cr2O3
Supporter Ratio ABET/(m2·g-1) vpore /(cm3·g-1) dpore/nm Mesoporosity /% vmicro vmeso vtotal dmicro dmeso HZSM-5 - 334 0.146 0.081 0.227 0.556 2.204 35.7 SAPO-11 - 225 0.102 0.075 0.177 0.566 5.194 42.4 Z+S 1:3.0 215 0.092 0.078 0.170 0.568 2.124 45.9 Z/S-6 1:1.8 208 0.089 0.087 0.176 0.542 2.151 49.4 Z/S-12 1:2.0 167 0.071 0.085 0.156 0.526 2.138 54.5 Z/S-24 1:2.3 167 0.072 0.085 0.157 0.525 2.151 54.1 mesoporosity: vmeso / vtotal 表 2 分子筛负载Cr2O3样品的NH3-TPD酸量
Table 2 NH3-TPD acid amounts of the molecular sieve supported Cr2O3
Sample Temperature t/℃ Acid amount /(mmol·g-1) Strong acid density/(mmol·m-2)×105 weak acid strong acid weak acid strong acid total HZSM-5 210 413 0.127 0.066 0.193 19.76 SAPO-11 200 398 0.250 0.005 0.255 2.22 Z+S 204 406 0.258 0.028 0.286 13.02 Z/S-6 208 472 0.315 0.016 0.331 7.69 Z/S-12 200 464 0.400 0.009 0.409 5.39 Z/S-24 209 475 0.389 0.012 0.401 7.19 -
[1] 金羽豪, 任文坡.甲醇制烯烃将成我国未来烯烃市场主流[J].中国石化, 2017, 1:31-33. http://www.cqvip.com/QK/98443X/201701/671480631.htmlJIN Yu-hao, REN Wen-po. Methanol to olefins will become the future olefins market mainstream of China[J]. Chin Pet Chem Ind, 2017, 1:31-33. http://www.cqvip.com/QK/98443X/201701/671480631.html [2] 丁郡瑜.中国煤制油产业现状与发展环境分析[J].国际石油经济, 2017, 25(4):45-49. http://d.wanfangdata.com.cn/Periodical/gjsyjj201704008DING Jun-yu. Status and development environment of China's CTL industry[J]. Int Pet Econ, 2017, 25(4):45-49. http://d.wanfangdata.com.cn/Periodical/gjsyjj201704008 [3] 阿古达木, 孙勇, 张飞跃.神华宁煤甲醇制丙烯装置产物分布探讨[J].煤化工, 2013, 1:58-60. doi: 10.3969/j.issn.1005-9598.2013.01.017A-Gu-da-mu, SUN-Yong, ZHANG Fei-yue. Discussion on product distribution of methanol to propylene in shenhua ningxia coal[J]. Coal Chem Ind, 2013, 1:58-60. doi: 10.3969/j.issn.1005-9598.2013.01.017 [4] 候庆贺, 杨靖华.液化石油气资源及其综合利用[J].当代化工, 2010, 39(3):287-289. http://d.wanfangdata.com.cn/Periodical/ddhg201003019HOU Qing-he, YANG Jing-hua. Liquefied petroleum gas resources and their comprehensive utilization[J]. Contemp Chem Ind, 2010, 39(3):287-289. http://d.wanfangdata.com.cn/Periodical/ddhg201003019 [5] HAJHEIDARY M, GHASHGHAEE M, KARIMZADEH R. Olefins production from LPG via dehydrogenative cracking over three ZSM-5 catalysts[J]. J Sci Ind Res, 2013, 72:760-766. https://www.researchgate.net/publication/288213226_Olefins_production_from_LPG_via_dehydrogenative_cracking_over_three_ZSM-5_catalysts [6] 王久昌, 张国良, 郝代军.液化石油气综合利用技术进展[J].炼油技术与工程, 2009, 39(10):1-6. doi: 10.3969/j.issn.1002-106X.2009.10.001WANG Jiu-chang, ZHANG Guo-liang, HAO Dai-jun. Advances in comprehensive utilization of liquefied petroleum gas[J]. Refin Technol Eng, 2009, 39(10):1-6. doi: 10.3969/j.issn.1002-106X.2009.10.001 [7] RAHIMI N, MORADI D, SHEIBAK M, MOOSAVI E, KARIMZADEH R. The influence of modification methods on the catalytic cracking of LPG over lanthanum and phosphorus modified HZSM-5 catalysts[J]. Microporous Mesoporous Mater, 2016, 234:215-223. doi: 10.1016/j.micromeso.2016.07.010 [8] VAFI L, KARIMZADEH R. A novel method for enhancing the stability of ZSM-5 zeolites used for catalytic cracking of LPG:Catalyst modification by dealumination and subsequent silicon loading[J]. Chin J Catal, 2016, 37:628-635. doi: 10.1016/S1872-2067(15)61062-2 [9] VAFI L, KARIMZADEH R. LPG catalytic cracking over the modified ZSM-5 by activated carbon and carbon nanotube templates:Synthesis, morphology and performance of catalysts[J]. J Nat Gas Sci Eng, 2016, 32:1-9. doi: 10.1016/j.jngse.2016.04.032 [10] KUMAR S M, HAMMER N, RØNNING M, HOLMEN A, CHEN D, WALMSLEY J C, ØYE G. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane:New insights by a comprehensive spectroscopic study[J]. J Catal, 2009, 261:116-128. doi: 10.1016/j.jcat.2008.11.014 [11] ZHANG P Q, GUO X W, GUO H C, WANG X S. Study of the performance of modified nano-scale ZSM-5 zeolite on olefins reduction in FCC gasoline[J]. J Mol Catal A:Chem, 2007, 261:139-146. doi: 10.1016/j.molcata.2006.08.012 [12] ZHAO G L, TENG J W, XIE Z K, JIN W Q, YANG W M, CHEN Q L, TANG Y. Effect of phosphorus on HZSM-5 catalyst for C4-olefin cracking reactions to produce propylene[J]. J Catal, 2007, 248:29-37. doi: 10.1016/j.jcat.2007.02.027 [13] KUBO K, IIDA H, NAMBA S, IGARASHI A. Comparison of steaming stability of Cu-ZSM-5 with those of Ag-ZSM-5, P/H-ZSM-5, and H-ZSM-5 zeolites as naphtha cracking catalysts to produce light olefin at high temperatures[J]. Appl Catal A:Gen, 2015, 489:272-279. doi: 10.1016/j.apcata.2014.10.041 [14] HOU X, QIU Y, ZHANG X, LIU G. Analysis of reaction pathways for n-pentane cracking over zeolites to produce light olefins[J]. Chem Eng J, 2017, 307:372-381. doi: 10.1016/j.cej.2016.08.047 [15] 王翠, 张瑞珍, 邢普, 温少波, 赵玲玲, 郭端阳, 赵亮富.碱处理和Zn改性对HZSM-5催化LPG芳构化性能的影响[J].天然气化工(C1化学与化工), 2015, 40(6):13-17. http://d.wanfangdata.com.cn/Periodical/trqhg201506003WANG Cui, ZHANG Rui-zhen, XING Pu, WEN Shao-bo, ZHAO Ling-ling, GUO Duan-yang, ZHAO Liang-fu. Effect of alkali treatment and Zn modification on catalytic performance of HZSM-5 for LPG aromatization[J]. Nat Gas Chem Ind, 2015, 40(6):13-17. http://d.wanfangdata.com.cn/Periodical/trqhg201506003 [16] CAEIRO G, CARVALHO R H, WANG X, LEMOS M A N D A, LEMOS F, GUISNET M, RIBEIRO R F, Activation of C2-C4 alkanes over acid and bifunctional zeolite catalysts[J]. J Mol Catal A:Chem, 2006, 255:131-158. doi: 10.1016/j.molcata.2006.03.068 [17] ZHU Q, KONDO J N., SETOYAMA T, YAMAGUCHI M, DOMENC K, TATSUMI T. Activation of hydrocarbons on acidic zeolites:superior selectivity of methylation of ethene with methanol to propene on weakly acidic catalysts[J]. Chem. Commun., 2008, 5164-5166. http://europepmc.org/abstract/MED/18956056 [18] WUO W, GUO W, XIAO W, LUO M. Dominant reaction pathway for methanol conversion to propene over high silicon H-ZSM-5[J]. Chem Eng Sci, 2011, 66:4722-4732. doi: 10.1016/j.ces.2011.06.036 [19] 詹金友, 张璐璐, 孙尧, 沈健.改性ZSM-5-SBA-15及甲苯甲醇烷基化性能研究[J].燃料化学学报, 2016, 44(4):489-494. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18818.shtmlZHAN Jin-you, ZHANG Lu-lu, SUN Yao, SHEN Jian. Modified ZSM-5-SBA-15 and its alkylation performance for toluene with methanol[J]. J Fuel Chem Technol, 2016, 44(4):489-494. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18818.shtml [20] FAN S, ZHOU J, LV J, LIU M, HUANG H, ZHANG J, ZHAO T S. Composite HZSM-5 with nanosheets for higher light olefin selectivity and longer lifetime in catalytic cracking mixed light hydrocarbons[J]. Chem Lett, 2015, 44:1697-1699. doi: 10.1246/cl.150816 [21] KOOHSARYAN E, ANBIA M. Nanosized and hierarchical zeolites:A short review[J]. Chin J Catal, 2016, 37:447-467. doi: 10.1016/S1872-2067(15)61038-5 [22] 吕江江, 黄星亮, 赵蕾蕾, 孙仁山, 胡龙旺, 龚艳.酸碱处理对ZSM-5分子筛物化性质和反应性能的影响[J].燃料化学学报, 2016, 44(6):732-737. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18852.shtmlLV Jiang-jiang, HUANG Xing-liang, ZHAO Lei-lei, SUN Ren-shan, HU Long-wang, GONG Yan. Effects of acid-alkali treatment on properties and reactivity of ZSM-5 catalyst[J]. J Fuel Chem Technol, 2016, 44(6):732-737. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18852.shtml [23] ZHANG Q, LI C, XU S, SHAN H, YANG C. Synthesis of a ZSM-5(core)/SAPO-5(shell) composite and its application in FCC[J]. J Porous Mater, 2013, 20:171-176. doi: 10.1007/s10934-012-9586-x [24] ZHANG X, WANG J W, ZHONG J, LIU A S, GAO J K. Characterization and catalytic performance of SAPO-11/Hβ composite molecular sieve compared with the mechanical mixture[J]. Microporous Mesoporous Mater, 2008, 108:13-21. doi: 10.1016/j.micromeso.2007.03.022 [25] 周志华, 鲁金明, 巫树峰, 周敬林, 王金渠.两步晶化法制备MCM-48/ZSM-5复合分子筛[J].无机材料学报, 2009, 24(2):325-329. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=wgcl200902025&dbname=CJFD&dbcode=CJFQZHOU Zhi-hua, LU Jin-ming, WU Shu-feng, ZHOU Jing-lin, WANG Jin-qu. Synthesis of MCM-48/ZSM-5 composite molecular sieve by two-step crystallization[J]. J Inorg Mater, 2009, 24(2):325-329. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=wgcl200902025&dbname=CJFD&dbcode=CJFQ [26] DUAN C, ZHANG X, ZHOU R, HUA Y, ZHANG L, CHEN J. Comparative studies of ethanol to propylene over HZSM-5/SAPO-34 catalysts prepared by hydrothermal synthesis and physical mixture[J]. Fuel Proc Technol, 2013, 108:31-40. doi: 10.1016/j.fuproc.2012.03.015 [27] WU G, WU W, WANG X, ZAN W, WANG W J, LI C. Nanosized ZSM-5 zeolites:Seed-induced synthesis and the relation between the physicochemical properties and the catalytic performance in the alkylation of naphthalene[J]. Microporous Mesoporous Mater, 2013, 180:187-195. doi: 10.1016/j.micromeso.2012.11.011 [28] JANSEN J C, GAAG F J, BEKKUM H. Identification of ZSM-type and other 5-ring containing zeolites by i.r. spectroscopy[J]. zeolites, 1984, 4:369-372. doi: 10.1016/0144-2449(84)90013-7 [29] CHAE H J, SONG Y H, JEONG K E, KIM C U, JEONG S Y. Physicochemical characteristics of ZSM-5/SAPO-34 composite catalyst for MTO reaction[J]. J Phys Chem Solids, 2010, 71:600-603. doi: 10.1016/j.jpcs.2009.12.046 [30] YANG Y, SUN C, DU J M, YUE Y H, HUA W M, ZHANG C L, SHEN W, XU H L.The synthesis of endurable B-Al-ZSM-5 catalysts with tunable acidity for methanol to propylene reaction[J]. Catal Commun, 2012, 24:44-47. doi: 10.1016/j.catcom.2012.03.013 [31] MULLER S, LIU Y, VISHNUVARTHAN M, SUN X Y, VEEN A C, HALLER G L, SANCHEZ M, LERCHER J A. Coke formation and deactivation pathways on HZSM-5 in the conversion of methanol to olefins[J]. Chem Eng J, 2015, 278:159-165. doi: 10.1016/j.cej.2014.11.026 [32] NIEMINEN V, KUMAR N, HEIKKILA T, LAINE E, VILLEGAS J, SALMI T, MURZIN Y D. Isomerization of 1-butene over SAPO-11 catalysts synthesized by varying synthesis time and silica sources[J]. Appl Catal A:Gen, 2004, 259:227-234. doi: 10.1016/j.apcata.2003.09.038 [33] NAKASAKA Y, NISHIMURA J I, TAGO T, MASUDA T. Deactivation mechanism of MFI-type zeolites by coke formation during n-hexane cracking[J]. J Catal, 2015, 278:159-165. http://www.sciencedirect.com/science/article/pii/S1385894714014697 -





 下载:
下载: