-
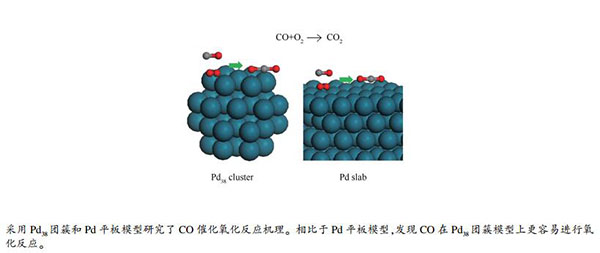
摘要: 采用密度泛函理论(DFT)计算模拟Pd平板和Pd38团簇上的CO催化氧化过程,分析了CO在Pd催化剂表面上的氧化反应机理。结果表明,在Pd38团簇模型上CO催化氧化的决速步骤是O2的解离,反应能垒为0.65 eV,而在Pd平板模型上的决速步骤是CO的氧化,其反应能垒为0.87 eV。对比决速步骤的活化能发现,CO在Pd38团簇上的氧化反应更易进行,说明CO氧化更易在小颗粒催化剂表面上进行,即Pd催化剂的活性与活性组分颗粒大小相关,活性组分颗粒越小,暴露的活性位点越多,其催化活性也越高。Abstract: The catalytic oxidation of CO was comparatively investigated on the Pd slab and Pd38 cluster models by density functional theory (DFT) calculation, in order to reveal the mechanism of CO oxidation over Pd catalysts. The results show that the rate-determining step of CO oxidation on the Pd38 cluster is the dissociation of O2, with the energy barrier of 0.65 eV, whereas the oxidation of CO turns to be the rate-determining step on Pd slab, with the energy barrier of 0.87 eV. Obviously, the oxidation of CO on the Pd38 cluster is much easier than that on the Pd slab, suggesting that the activity of Pd catalysts is related to the dispersion of active Pd species; the Pd catalyst with higher Pd dispersion also exhibits higher activity in CO oxidation.
-
表 1 CO与其反应中间物在Pd模型上的吸附能与相应的结构参数
Table 1 Optimized geometric parameters and adsorption energies of possible adsorbates involved in CO oxidation on the Pd38 cluster and Pd slab
Configuration Adsorption energy E/eV Geometric parameters/nm CO-hcp/Pd38 -1.98 Pd1-C: 0.3088; Pd2-C: 0.3086; Pd3-C: 0.3084 CO-fcc/Pd38 -1.96 Pd1-C: 0.3130; Pd2-C: 0.3132; Pd3-C: 0.3130 CO-top/Pd38 -1.41 Pd-C: 0.1911 CO-edge/Pd38 -1.22 Pd-C: 0.2008 O2/Pd38 -0.89 Pd-O1: 0.2021; Pd-O2: 0.2024 O-hcp/Pd38 -3.63 Pd1-O: 0.2019; Pd2-O: 0.2020; Pd3-O: 0.2024 O-bridge/Pd38 -2.59 Pd1-O: 0.1950; Pd2-O: 0.1948 CO2-bridge/Pd38 -0.39 Pd-C: 0.2134; Pd-O: 0.2096 CO2-edge/Pd38 -0.63 Pd-C: 0.2021 CO-top/Pd-slab -1.37 Pd-C: 0.1932 O2/Pd-slab -0.72 Pd-O1: 0.2030; Pd-O2: 0.2033 O-bridge/Pd-slab -3.15 Pd1-O: 0.1981; Pd2-O: 0.1980 O-hcp/Pd-slab -3.27 Pd1-O: 0.2034; Pd2-O: 0.2033; Pd3-O: 0.2034 CO2/Pd-slab -0.27 Pd-C: 0.2167; Pd-O: 0.2122 表 2 CO在Pd模型上基元反应的活化能和反应能
Table 2 Activation energies and reaction energies of the elementary steps involved in CO oxidation on the Pd38 cluster and Pd slab
Configurations Elementary step Activation energy Ea/eV Reaction energy ΔE/eV O2 dissociation/Pd38 O2 → 2O 0.65 -0.48 CO oxidation-1/Pd38 CO + O → CO2 0.75 0.05 CO oxidation-2/Pd38 CO + O → CO2 0.51 -0.31 O2 dissociation/Pd-slab O2 → 2O 0.79 -0.27 CO oxidation/Pd-slab CO + O → CO2 0.87 0.24 -
[1] KIEKEN L D, NEUROCK M, MEID H. Screening by kinetic monte carlo simulation of Pt-Au(100) surfaces for the steady-state decomposition of nitric oxide in excess dioxygen[J]. J Phys Chem B, 2005, 109(6):2234-2244. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f4297eceb58e5567581681eb50a14288 [2] GANDHI H S, GRAHAM G W, MCCABE R W. Automotive exhaust catalysis[J]. J Catal, 2003, 216(1/2):433-442. http://d.old.wanfangdata.com.cn/Periodical/gdyjs200702042 [3] CHENG X, SHI Z, GLASS N, ZHANG L, ZHANG J J, SONG D T, LIU Z S, WANG H J, SHEN J. A review of PEM hydrogen fuel cell contamination:Impacts, mechanisms, and mitigation[J]. J Power Sources, 2007, 165(2):739-756. doi: 10.1016/j.jpowsour.2006.12.012 [4] WANG S, ANG H M, TADE M O. Volatile organic compounds in indoor environment and photocatalytic oxidation:State of the art[J]. Environ Int, 2007, 33(5):694-705. doi: 10.1016/j.envint.2007.02.011 [5] KAGEYAMA S, SUGANO Y, HAMAGUCHI Y, KUGAI J, OHKUBO Y, SEINO S, NAKAGAWA T, ICHIKAWA S, YAMAMOTO T A. Pt/TiO2 composite nanoparticles synthesized by electron beam irradiation for preferential CO oxidation[J]. Mater Res Bull, 2013, 48(4):1347-1351. doi: 10.1016/j.materresbull.2012.11.028 [6] ATES A, PFEIFER P, GOERKE O. Thin-Film catalytic coating of a microreactor for preferential CO oxidation over Pt catalysts[J]. Chem Ing Tech, 2013, 85(5):664-672. doi: 10.1002/cite.201200166 [7] GARCIA-DIEGUEZ M, IGLESIA E. Structure sensitivity via decoration of low-coordination exposed metal atoms:CO oxidation catalysis on Pt clusters[J]. J Catal, 2013, 301:198-209. doi: 10.1016/j.jcat.2013.02.014 [8] LI Y Z, YU Y, WANG J G, SONG J, LI Q, DONG M D, LIU C J. CO oxidation over graphene supported palladium catalyst[J]. Appl Catal B:Environ, 2012, 125:189-196. doi: 10.1016/j.apcatb.2012.05.023 [9] LIU L Q, ZHOU F, WANG L G, QI X J, SHI F, DENG Y Q. Low-temperature CO oxidation over supported Pt, Pd catalysts:Particular role of FeOx support for oxygen supply during reactions[J]. J Catal, 2010, 274(1):1-10. [10] TODOROKI N, OSANO H, MAEYAMA T, YOSHIDA H, WADAYAMA T. Infrared reflection absorption spectral study for CO adsorption on Pd/Pt(111) bimetallic surfaces[J]. Appl Surf Sci, 2009, 256(4):943-947. doi: 10.1016/j.apsusc.2009.05.070 [11] JAATINEN S, SALO P, ALATALO M, KULMALA V, KOKKO K. Structure and reactivity of Pd doped Ag surfaces[J]. Surf Sci, 2003, 529(3):403-409. doi: 10.1016/S0039-6028(03)00304-2 [12] DESAI S K, NEUROCK M. First-principles study of the role of solvent in the dissociation of water over a Pt-Ru alloy[J]. Phys Rev B, 2003, 68(7):075420. doi: 10.1103/PhysRevB.68.075420 [13] DESAI S K, NEUROCK M. A first principles analysis of CO oxidation over Pt and Pt66.7%Ru33.3% (111) surfaces[J]. Electrochim Acta, 2003, 48(25/26):3759-3773. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8f934a0ca79cacdcc4cfa0378497f04c [14] SUO Y G, ZHUANG L, LU J T. First-principles considerations in the design of Pd-alloy catalysts for oxygen reduction[J]. Angew Chem Int Ed, 2007, 46(16):2862-2864. doi: 10.1002/anie.200604332 [15] YAO Y F Y. The oxidation of CO and hydrocarbons over noble-metal catalysts[J]. J Catal, 1984, 87(11):152-162. http://cn.bing.com/academic/profile?id=c51f45fa37418adba9c0c493317af2be&encoded=0&v=paper_preview&mkt=zh-cn [16] ERTL G. Oscillatory kinetics and spatiotemporal self-organization in reactions at solid-surfaces[J]. Science, 1991, 254(5039):1750-1755. doi: 10.1126/science.254.5039.1750 [17] LIAN X, GUO W L, LIU F L, YANG Y, XIAO P, ZHANG Y H, TIAN W Q. DFT studies on Pt3M(M=Pt, Ni, Mo, Ru, Pd, Rh) clusters for CO oxidation[J]. Comput Mater Sci, 2015, 96:237-245. doi: 10.1016/j.commatsci.2014.09.025 [18] KRAUSA M, VIELSTICH W. Potential oscillations during methanol oxidation at Pt-electrodes.1. Experimental conditions[J]. J Electroanal Chem, 1995, 399(1/2):7-12. doi: 10.1016-0022-0728(95)04249-0/ [19] TONG Y Y, KIM H S, BABU P K, WASZCZUK P, WIECKOWSKI A, OLDFIELD E. An NMR investigation of CO tolerance in a Pt/Ru fuel cell catalyst[J]. J Am Chem Soc, 2002, 124(3):468-473. doi: 10.1021/ja011729q [20] DAVIES J C, BONDE J, LOGADOTTIR A, NORSKOV J K, CHORKENDORFF I. The ligand effect:CO desorption from Pt/Ru catalysts[J]. Fuel Cell, 2005, 5(4):429-435. http://cn.bing.com/academic/profile?id=d50978e820e183a1cf858fc98454726a&encoded=0&v=paper_preview&mkt=zh-cn [21] WANG F G, XU Y, ZHAO K F, HE D N. Preparation of palladium supported on ferric oxide nano-catalysts for carbon monoxide oxidation in low temperature[J]. Nano-Micro Lett, 2014, 6(3):233-241. doi: 10.1007/BF03353787 [22] PARK R L, SCHREINE D. Oxidation of carbon-monoxide on palladium[J]. J Vac Sci Technol, 1974, 11(1):248-248. doi: 10.1116/1.1318581 [23] ENGEL T, ERTL G. Surface residence times and reaction-mechanism in catalytic-oxidation of CO on Pd(111)[J]. Chem Phys Lett, 1978, 54(1):95-98. http://cn.bing.com/academic/profile?id=8f3fedb70cf03fe5727e80e9aa096fec&encoded=0&v=paper_preview&mkt=zh-cn [24] MURATA K, ELEEDA E, OHYAMA J, YAMAMOTO Y, ARAI S, SATSUMA A. Identification of active sites in CO oxidation over a Pd/Al2O3 catalyst[J]. Phys Chem Chem Phys, 2019, 21(33):18128-18137. doi: 10.1039/C9CP03943K [25] LI S Y, LIU G, LIAN H L, JIA M J, ZHAO G M, JIANG D Z, ZHANG W X, Low-temperature CO oxidation over supported Pt catalysts prepared by colloid-deposition method[J]. Catal Commun, 2008, 9(6):1045-1049. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=59f7e79367b9c439d87e6c614eaa1e5d [26] TELKAR M M, RODE C V, CHAUDHARI R V, JOSHI S S, NALAWADE A M. Shape-controlled preparation and catalytic activity of metal nanoparticles for hydrogenation of 2-butyne-1, 4-diol and styrene oxide[J]. Appl Catal A:Gen, 2004, 273(1/2):11-19. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=51472866f67c81c9bdb25a10a307157a [27] HUANG H H, NI X P, LOY G L, CHEW C H, TAN K L, LOH F C, DENG J F, XU G Q. Photochemical formation of silver nanoparticles in poly(N-vinylpyrrolidone)[J]. Langmuir, 1996, 12(4):909-912. http://cn.bing.com/academic/profile?id=d1863c42bb8e1cba00ec703385256b5f&encoded=0&v=paper_preview&mkt=zh-cn [28] SARKAR A, KAPOOR S, MUKHERJEE T. Preparation, characterization, and surface modification of silver nanoparticles in formamide[J]. J Phys Chem B, 2005, 109(16):7698-7704. doi: 10.1021/jp044201r [29] GUNAY M E, YILDIRIM R. Modeling preferential CO oxidation over promoted Au/Al2O3 catalysts using decision trees and modular neural networks[J]. Chem Eng Res Des, 2013, 91(5):874-882. doi: 10.1016/j.cherd.2012.08.017 [30] KUGAI J, MORIYA T, SEINO S, NAKAGAWA T, OHKUBO Y, NITANI H, YAMAMOTO T A. Comparison of structure and catalytic performance of Pt-Co and Pt-Cu bimetallic catalysts supported on Al2O3 and CeO2 synthesized by electron beam irradiation method for preferential CO oxidation[J]. Int J Hydrogen Energy, 2013, 38(11):4456-4465. doi: 10.1016/j.ijhydene.2013.01.159 [31] GILROY K D, RUDISKIY A, PENG H C, QIN D, XIA Y N. Bimetallic nanocrystals:Syntheses, properties, and applications[J]. Chem Rev, 2016, 116(18):10414-10472. doi: 10.1021/acs.chemrev.6b00211 [32] HUTCHINGS G J, KIELY C J. Strategies for the synthesis of supported gold palladium nanoparticles with controlled morphology and composition[J]. Acc Chem Res, 2013, 46(8):1759-1772. doi: 10.1021/ar300356m [33] JACQUES S D M, MICHIEL M D, BEALE A M, SOCHI T, O'BRIEN M G, ESPINOSA-ALONSO L, WECKHUYSEN B M, BARNES P. Dynamic X-ray diffraction computed tomography reveals real-time insight into catalyst active phase evolution[J]. Angew Chem Int Ed, 2011, 50(43):10148-10152. doi: 10.1002/anie.201104604 [34] BANGER K K, YAMASHITA Y, MORI K, PETERSON R L, LEEDHAM T, RICKARD J, SIRRINGHAUS H. Low-temperature, high-performance solution-processed metal oxide thin-film transistors formed by a 'sol-gel on chip' process[J]. Nat Mater, 2011, 10(1):45-50. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ff1038b8d89f7fde8e2342406a1bfeba [35] UCHIYAMA T, YOSHIDA H, KUWAUCHI Y, ICHIKAWA S, SHIMADA S, HARUTA M, TAKEDA S. Systematic morphology changes of gold nanoparticles supported on CeO2 during CO oxidation[J]. Angew Chem Int Ed, 2011, 50(43):10157-10160. doi: 10.1002/anie.201102487 [36] ZHANG J, JIN H, SULLIVAN M B, CHIANG F, LIM H, WU P. Study of Pd-Au bimetallic catalysts for CO oxidation reaction by DFT calculations[J]. Phys Chem Chem Phys, 2009, 11(9):1441-1446. doi: 10.1039/b814647k [37] HUBER B, MOSELER M. Predicting experimental signatures for the oxidation of magnesia supported palladium clusters by density functional theory[J]. Eur Phys J D, 2007, 45(3):485-489. doi: 10.1140/epjd/e2007-00178-5 [38] KALITA B, DEKA R C. DFT study of CO adsorption on neutral and charged Pd-n(n=1-7) clusters[J]. Eur Phys J D, 2009, 53:51-58. doi: 10.1140/epjd/e2009-00044-6 [39] KALITA B, DEKA R C. Reaction intermediates of CO oxidation on gas phase Pd-4 clusters:A density functional study[J]. J Am Chem Soc, 2009, 131(37):13252-13254. doi: 10.1021/ja904119b [40] CHEN H, WU Y, QI S, CHEN Y, YANG M. Deoxygenation of octanoic acid catalyzed by hollow spherical Ni/ZrO2[J]. Appl Catal A:Gen, 2017, 529:79-90. doi: 10.1016/j.apcata.2016.10.014 [41] WANG B, SONG L, ZHANG R. The dehydrogenation of CH4 on Rh(111), Rh(110) and Rh(100) surfaces:A density functional theory study[J]. Appl Surf Sci, 2012, 258(8):3714-3722. doi: 10.1016/j.apsusc.2011.12.012 [42] ZHANG R, SONG L, WANG Y. Insight into the adsorption and dissociation of CH4 on Pt(h k l) surfaces:A theoretical study[J]. Appl Surf Sci, 2012, 258(18):7154-7160. doi: 10.1016/j.apsusc.2012.04.020 [43] WANG D, LI Y. Bimetallic nanocrystals:Liquid-phase synthesis and catalytic applications[J]. Adv Mater, 2011, 23(9):1044-1060. doi: 10.1002/adma.201003695 -





 下载:
下载: