Study on the performance of low temperature NH3-SCR over MnO2 nano-catalyst with different crystal structures
-
摘要: 为了探究催化剂的结构和催化活性的关系,采用水热法制备了四种不同晶体结构的MnO2纳米催化剂(α-MnO2、β-MnO2、γ-MnO2和δ-MnO2),并考察了其低温NH3-SCR活性。结果表明,不同晶体结构催化剂的活性不同,依次为γ-MnO2 > α-MnO2 > β-MnO2 > δ-MnO2,γ-MnO2表现出最高的催化活性,NOx转化率在150-260℃超过90%。随后,通过X射线衍射(XRD)、扫描电子显微镜(SEM)、N2吸附-脱附、热重(TG)、红外光谱(FT-IR)、程序升温还原(H2-TPR)及吡啶吸附红外光谱(Py-FTIR)等表征手段对催化剂的结构和性质进行分析。结果表明,α-MnO2和β-MnO2为纳米棒,γ-MnO2和δ-MnO2为纳米针,催化剂的比表面积并不是影响低温NH3-SCR活性的主导因素。γ-MnO2具有适宜的孔道结构、较强的氧化还原能力、丰富的化学氧含量和Lewis酸酸性位点,是其具有最高低温NH3-SCR活性的原因。Abstract: To investigate the relationship between the structure and catalytic activity, four types of MnO2 nano-catalysts with various crystal structures (α-MnO2, β-MnO2, γ-MnO2 and δ-MnO2) were synthesized by hydrothermal method, and their low temperature NH3-SCR activity were tested. The results indicated that catalysts with different structures showed various activities which followed the sequence of γ-MnO2 > α-MnO2 > β-MnO2 > δ-MnO2. It was found that γ-MnO2 showed highest catalytic activity and its NOx conversion rate surpassed 90% at the temperature range of 150-260℃. The catalysts were characterized by X-ray diffraction(XRD), scanning electron microscopy (SEM), N2 adsorption-desorption, thermogravimetric(TG), infrared (FT-IR), temperature programmed reduction(H2-TPR) and pyridine infrared spectroscopy (Py-FTIR). It was inferred that the morphology of the α-MnO2 and β-MnO2 were nanorods, while γ-MnO2 and δ-MnO2 with the structures of nanoneedles. The specific surface area of the catalyst was not the dominant factor affecting the NH3-SCR activity at low temperature. The decent pore structure, strong redox property, abundant chemisorption oxygen and Lewis acid sites were responsible for high low temperature NH3-SCR activity of γ-MnO2 nano-catalyst.
-
Key words:
- hydrothermal method /
- MnO2 nano-catalyst /
- crystal structure /
- low temperature NH3-SCR
-
表 1 α-MnO2、β-MnO2、γ-MnO2和δ-MnO2催化剂的孔容、孔径和比表面积
Table 1 Specific surface area, pore volume and pore diameter distribution of the α-MnO2, β-MnO2, γ-MnO2 and δ-MnO2catalysts
Catalyst BET surface areas A/(m2·g-1) Total pore volume v/(cm3·g-1) Average pore size d/nm α-MnO2 84.760 0.189 16.692 β-MnO2 32.383 0.045 20.762 γ-MnO2 104.75 0.258 18.669 δ-MnO2 79.765 0.213 16.534 -
[1] PÂRVULESCU V I, GRANGE P, DELMON B. Catalytic removal of NO[J]. Catal Today, 1998, 46(4):233-316. doi: 10.1016/S0920-5861(98)00399-X [2] ROY S, HEGDE MS, MADRAS G. Catalysis for NOx abatement[J]. Appl Energy, 2009, 86(11):2283-2297. doi: 10.1016/j.apenergy.2009.03.022 [3] LIU C, SHI J-W, GAO C, NIU C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3:A review[J]. Appl Catal A:Gen, 2016, 522:54-69. doi: 10.1016/j.apcata.2016.04.023 [4] 杨永利, 徐东耀, 晁春艳, 高明.负载型Mn基低温NH3-SCR脱硝催化剂研究综述[J].化工进展, 2016, 35(4):1094-1100. http://www.chxb.cn/CN/abstract/abstract21882.shtmlYANG Yong-li, XU Dong-yao, CHAO Chun-yan, GAO Ming. Research advance review on supported Mn-based catalysts at low-temperature selective catalytic reduction of NOx with NH3[J]. Chem Ind Eng Prog, 2016, 35(4):1094-1100. http://www.chxb.cn/CN/abstract/abstract21882.shtml [5] 刘亭, 王廷春, 吴瑞青, 沈伯雄.低温NH3-SCR脱硝催化剂研究进展[J].安全与环境学报, 2012, 19(6):42-44. http://www.chxb.cn/CN/abstract/abstract21645.shtmlLIU Ting, WANG Ting-chun, WU Rui-qing, SHEN Bo-xiong.Research advance review for low-temperature NH3 -SCR catalysts[J]. J Saf Environ, 2012, 19(6):42-44. http://www.chxb.cn/CN/abstract/abstract21645.shtml [6] KAPTEIJN F, SINGOREDJO L, ANDREINI A, MOULIJN J A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia[J]. Appl Catal B:Environ, 1994, 3(2/3):173-189. https://www.sciencedirect.com/science/article/pii/0926337393E00349 [7] 刘林林, 田华, 贺军辉, 杨巧文, 王东.隐钾锰矿型和水钠锰矿型氧化锰的研究进展[J].化学通报, 2011, 74(4):000291-000297. http://edu.wanfangdata.com.cn/Periodical/Detail/hxtb2201104001LIU Lin-lin, TIAN Hua, HE Jun-hui, YANG Qiao-wen, WANG Dong. Progress in cryptomelane-and birnessite manganese oxides[J]. Chem Bull, 2011, 74(4):000291-000297. http://edu.wanfangdata.com.cn/Periodical/Detail/hxtb2201104001 [8] THACKERAY M M. Manganese oxides for lithium batteries[J]. Prog Solid State Chem, 1997, 25(1/2):1-71. https://www.sciencedirect.com/science/article/pii/S0079678697810035 [9] LIANG S, TENG F, BULGAN G, RUILONG ZONG A, ZHU Y. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation[J]. J Phys Chem C, 2010, 112(14):5307-5315. http://cn.bing.com/academic/profile?id=b8d1c35f0ad73d0faaa95d2110432375&encoded=0&v=paper_preview&mkt=zh-cn [10] ZHANG J, LI Y, WANG L, ZHANG C, HE H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures[J]. Catal Sci Technol, 2015, 5(4):2305-2313. doi: 10.1039/C4CY01461H [11] 戴韵, 李俊华, 彭悦, 唐幸福. MnO2的晶相结构和表面性质对低温NH3-SCR反应的影响[J].物理化学学报, 2012, 28(7):1771-1776. http://www.whxb.pku.edu.cn/CN/abstract/abstract28056.shtmlDAI Yun, LI Jun-hua, PENG Yue, TANG Xing-fu. Effects of MnO2 crystal structure and surface property on the NH3-SCR reaction at low temperature[J]. Acta Phys Chim Sin, 2012, 28(7):1771-1776. http://www.whxb.pku.edu.cn/CN/abstract/abstract28056.shtml [12] LI Y, LI Y, WAN Y, ZHAN S, GUAN Q, TIAN Y. Structure-performance relationships of MnO2nanocatalyst for the low-temperature SCR removal of NOx under ammonia[J]. RSC Adv, 2016, 6(60):54926-54937. doi: 10.1039/C6RA03108K [13] 孙梦婷, 黄碧纯, 马杰文, 李时卉, 董立夫.二氧化锰在低温NH3-SCR催化反应上的形貌效应[J].物理化学学报, 2016, 32(6):1501-1510. doi: 10.3866/PKU.WHXB201603171SUN Meng-ting, HUANG Bi-chun, MA Jie-wen, LI Shi-hui, DONG Li-fu. Morphological effects of manganese dioxide on catalytic reactions for low-temperature NH3-SCR[J]. Acta Phys Chim Sin, 2016, 32(6):1501-1510. doi: 10.3866/PKU.WHXB201603171 [14] SUN M, LAN B, YU L, YE F, SONG W, HE J, DIAO G, ZHENG Y. Manganese oxides with different crystalline structures:Facile hydrothermal synthesis and catalytic activities[J]. Mater Lett, 2012, 86:18-20. doi: 10.1016/j.matlet.2012.07.011 [15] 王燕彩, 刘昕, 宁平, 张秋林, 张金辉, 徐利斯, 唐小苏, 王明智.制备方法对氧化锰八面体分子筛的NH3选择性催化还原NOx性能的影响[J].燃料化学学报, 2014, 42(11):1357-1364. doi: 10.3969/j.issn.0253-2409.2014.11.013WANG Yan-cai, LIU Xin, NING Ping, ZHANG Qiu-lin, ZHANG Jin-hui, XU Li-si, TANG Xiao-su, WANG Ming-zhi. Effect of preparation methods on selective catalytic reduction of NOx with NH3 over manganese oxide octahedral molecular sieves[J]. J Fuel Chem Technol, 2014, 42(11):1357-1364. doi: 10.3969/j.issn.0253-2409.2014.11.013 [16] 苏潜, 黄妍, 张颖, 李元元, 唐南, 张俊丰, 杨柳春.铜源对Cu-S APO-34氨催化还原NO性能的影响[J].分子催化, 2016, 30(2):151-158. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fzch201602007SU Qian, HUANG Yan, ZHANG Yin, LI Yuan-yuan, TANG Nan, ZHANG Jun-fen, YANG Liu-chun. Effects of copper sources on selective catalytic reduction of NO with NH3 of Cu-SAPO-34[J]. J Mol Catal, 2016, 30(2):151-158. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fzch201602007 [17] GIOVANOLI R. Thermogravimetry of manganese dioxides[J]. Thermochim Acta, 1994, 234(94):303-313. https://www.sciencedirect.com/science/article/pii/0040603194851549 [18] WANG C, SUN L, CAO Q, HU B, HUANG Z, TANG X. Surface structure sensitivity of manganese oxides for low-temperature selective catalytic reduction of NO with NH3[J]. Appl Catal B:Environ, 2011, 101(3/4):598-605. doi: 10.1016/j.apcatb.2010.10.034 [19] WANG ZM, KANOH H. Calorimetric study on NH3 insertion reaction into microporous manganese oxides with (2×2) tunnel and (2×∞) layered structures[J]. Thermochim Acta, 2001, 379(1):7-14. https://www.sciencedirect.com/science/article/pii/S0040603101005962 [20] 郭学益, 刘海涵, 李栋, 田庆华, 徐刚.二氧化锰晶型转变研究[J].矿冶工程, 2007, 27(1):50-53. http://jz.docin.com/p-1236768287.htmlGUO Xue-yi, LIU Hai-han, LI Dong, TIAN Qing-hua, XU Gang. Influence of thermal treatment on the crystal phase transformation of MnO2[J]. Min Metal Eng, 2007, 27(1):50-53. http://jz.docin.com/p-1236768287.html [21] AND S D, MUNICHANDRAIAH N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties[J]. J Phys Chem C, 2008, 112(11):4406-4417. doi: 10.1021/jp7108785 [22] LI Y, FAN Z, SHI J, LIU Z, ZHOU J, SHANGGUAN W. Modified manganese oxide octahedral molecular sieves M'-OMS-2(M'=Co, Ce, Cu) as catalysts in post plasma-catalysis for acetaldehyde degradation[J]. Catal Today, 2015, 256:178-185. doi: 10.1016/j.cattod.2015.02.003 [23] XIE J, CHEN L, ZHOU W F, AU C T, YIN S F. Selective oxidation of p-chlorotoluene to p-chlorobenzaldehyde over metal-modified OMS-2 molecular sieves[J]. J Mol Catal A:Chem, 2016, 425:110-115. doi: 10.1016/j.molcata.2016.09.038 [24] 辛勤, 罗孟飞.现代催化研究方法[M].北京:科学出版社, 2009.XIN Qin, LUO Meng-fei. The Modern Catalytic Research Method[M]. Beijing:Science Press, 2009. [25] WU Z, JIANG B, LIU Y, WANG H, JIN R. DRIFT study of manganese/titania-based catalysts for low-temperature selective catalytic reduction of NO with NH3[J]. Environ Sci Technol, 2007, 41(16):5812-5817. doi: 10.1021/es0700350 [26] YU C, HUANG B, DONG L, CHEN F, LIU X. In situ FT-IR study of highly dispersed MnOx/SAPO-34 catalyst for low-temperature selective catalytic reduction of NOx by NH3[J]. Catal Today, 2017, 281:610-620. doi: 10.1016/j.cattod.2016.06.025 -
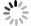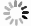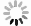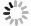




 下载:
下载: