Determination of mass transfer behavior of typical products of MTO (methanol to olefins) reactions over HZSM-5 zeolite
-
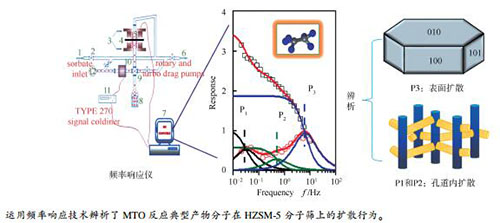
摘要: 分子筛催化甲醇制烯烃反应(MTO)是典型的扩散主导反应过程,运用频率响应技术系统研究了几种典型产物分子(乙烯/乙烷、丙烯/丙烷、苯)在HZSM-5分子筛上的扩散行为。结果表明,频率响应法成功辨析了不同产物分子的传质规律,证实C2和C3烃分子在HZSM-5微孔孔道内具有相近的扩散速率,但由于受晶体表面阻碍效应影响不同,乙烷分子可自由进出HZSM-5分子筛孔道,而丙烷分子则受到较显著的微孔孔道扩散限制。另外,苯分子的扩散速率显著小于C2和C3分子,且苯分子受晶体表面阻抗效应的影响较小。本研究结果可用于解释HZSM-5分子筛在MTO反应中产物选择性的特点及表面结焦原因,进而从传质角度为高活性、选择性以及稳定性的高效甲醇转化制烃催化剂的定向开发提供理论指导。Abstract: Methanol conversion to olefins (MTO) catalyzed by zeolite catalysts is a typical diffusion dominated reaction process. In this paper, the diffusion behavior of several typical product molecules (ethylene/ethane, propylene/propane, benzene) on a HZSM-5 zeolite was systematically studied by using Frequency Response method. The results show that the mass transfer regularity of the product molecules have been successfully determined by the Frequency Response method. It is confirmed that the diffusion rates of C2 and C3 hydrocarbon molecules within the HZSM -5 micropores are similar, but the effects of the surface resistance are different. So, the C2 molecules can freely go in and out of the channels of the HZSM-5 zeolite, while the diffusion of C3 molecules is significantly affected by the channel diffusion limitation. In addition, the diffusion rate of benzene molecules is observably lower than that of C2 and C3 molecules, and the resistant effects of benzene molecules caused by the zeolite crystal surface are not serious. The conclusions obtained in this study can be used to explain the product selectivity of MTO reaction over HZSM-5 zeolites and the coking mechanism of the catalyst, and provide the mass transfer theoretical guidance for the preparation of the MTO catalysts with excellent performance.
-
Key words:
- HZSM-5 /
- methanol conversion /
- mass transfer behavior /
- frequency response method /
- surface barrier
-
图 1 频率响应装置示意图[27]
1: sorbate inlet; 2: valve; 3: electromagnet; 4: armature; 5: bellow; 6: rotary and turbo drag pump; 7: computer workstation; 8: sample cell; 9: vacuum flange connections; 10: pressure transducer; 11: type 270 signal conditioner; 12: pressure reference
Figure 1 Schematics of the frequency response apparatus[27]
表 1 乙烯和乙烷在HZSM-5分子筛上传质过程的时间常数和响应强度值
Table 1 Time constant and response intensity values of ethene and ethane in the process of mass transfer in the HZSM-5 zeolite
p/Pa Ethylene Ethane f1/ s-1 f2/ s-1 K1 K2 f1/ s-1 f2/ s-1 K1 K2 66 0.06 15.9 0.012 0.19 0.05 12.48 0.009 0.085 133 0.08 27.1 0.018 0.15 0.047 12.28 0.009 0.07 表 2 丙烯和丙烷在HZSM-5分子筛上传质过程的时间常数和响应强度值
Table 2 Time constant and response intensity values of propene and propane in the process of mass transfer in the HZSM-5 zeolite
p/Pa Propylene Propane f1/ s-1 f2/ s-1 f3/ s-1 K1 K2 K3 f1/ s-1 f2/ s-1 f3/ s-1 K1 K2 K3 66 0.08 0.80 15.93 0.09 0.07 0.67 0.03 0.51 6.43 1.05 0.57 1.87 133 0.06 0.48 35.04 0.04 0.03 0.44 0.03 0.88 15.92 0.69 0.41 1.15 表 3 苯在HZSM-5分子筛上传质过程的时间常数和响应强度值
Table 3 Time constant and response intensity values of benzene in the process of mass transfer in the HZSM-5 zeolite
p/Pa Benzene f1/ s-1 f2/ s-1 K1 K2 66 0.03 1.84 0.47 0.09 133 0.036 0.48 0.23 0.08 -
[1] CHANG C D. Hydrocarbons from methanol[J]. Catal Rev, 1983, 25(1):1-118. doi: 10.1080/01614948308078874 [2] CHANG C D. Methanol conversion to light olefins[J]. Catal Rev, 1984, 26(3/4):323-345. http://d.old.wanfangdata.com.cn/Periodical/sxhg201803035 [3] SUN Q, XIE Z K, YU J. The state-of-the-art synthetic strategies for SAPO-34 zeolite catalysts in methanol-to-olefin conversion[J]. Natl Sci Rev, 2018, 5(4):542-558. doi: 10.1093/nsr/nwx103 [4] DAHL I M, KOLBOE S. On the reaction mechanism for propene formation in the MTO reaction over SAPO-34[J]. Catal Lett, 1993, 20(3/4):329-336. doi: 10.1007-BF00769305/ [5] LI J, WEI Y, LIU G, QI Y, TIAN P, LI B, HE Y, LIU Z. Comparative study of MTO conversion over SAPO-34, H-ZSM-5 and H-ZSM-22:Correlating catalytic performance and reaction mechanism to zeolite topology[J]. Catal Today, 2011, 171(1):221-228. doi: 10.1016/j.cattod.2011.02.027 [6] 王森, 陈艳艳, 卫智虹, 秦张峰, 李俊汾, 董梅, 樊卫斌, 王建国.分子筛骨架结构和酸性对其甲醇制烯烃(MTO)催化性能影响研究进展[J].燃料化学学报, 2015, 43(10):1202-1214. doi: 10.3969/j.issn.0253-2409.2015.10.008WANG Sen, CHEN Yan-yan, WEI Zhi-hong, QIN Zhang-feng, LI Jun-fen, DONG Mei, FAN Wei-bin, WANG Jian-guo. Recent research progresses in the effect of framework structure and acidity of zeolites on their catalytic performance in methanol to olefins (MTO)[J]. J Fuel Chem Technol, 2015, 43(10):1202-1214. doi: 10.3969/j.issn.0253-2409.2015.10.008 [7] BJØRGEN M, SVELLE S, JOENSEN F, NERLOV J, BONINO F, PALUMBO L, BORDIGA S, OLSBYE U. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5:On the origin of the olefinic species[J]. J Catal, 2007, 249(2):195-207. http://www.sciencedirect.com/science/article/pii/S002195170700156X [8] BLEKEN F L, CHAVAN S, OLSBYE U, BOLTZ M, OCAMPO F, LOUIS B. Conversion of methanol into light olefins over ZSM-5 zeolite:Strategy to enhance propene selectivity[J]. Appl Catal A:Gen, 2012, 447:178-185. http://cn.bing.com/academic/profile?id=f179f599102efd1d4f599c90f2ffbd3e&encoded=0&v=paper_preview&mkt=zh-cn [9] 张立伟, 张怀科, 陈志强, 刘粟侥, 任杰. ZSM-5分子筛骨架铝落位对甲醇转化制芳烃催化性能影响[J].燃料化学学报, 2019, 47(12):1468-1475. doi: 10.3969/j.issn.0253-2409.2019.12.007ZHANG Li-wei, ZHANG Huai-ke, CHEN Zhi-qiang, LIU Su-yao, REN Jie. Effect of framework Al siting on catalytic performance in methanol to aromatics over ZSM-5 zeolites[J]. J Fuel Chem Technol, 2019, 47(12):1468-1475. doi: 10.3969/j.issn.0253-2409.2019.12.007 [10] FIROOZI M, BAGHALHA M, ASADI M. The effect of micro and nano particle sizes of H-ZSM-5 on the selectivity of MTP reaction[J]. Catal Commun, 2009, 10(12):1582-1585. doi: 10.1016/j.catcom.2009.04.021 [11] 张云鹏, 李明罡, 邢恩会, 罗一斌, 舒兴田.不同结构扩孔分子筛催化MTP反应行为及表面积炭物种表征[J].燃料化学学报, 2018, 46(9):1101-1112. doi: 10.3969/j.issn.0253-2409.2018.09.009ZHANG Yun-peng, LI Ming-gang, XING En-hui, LUO Yi-bin, SHU Xing-tian. Methanol to propylene reaction performance and trapped carbonaceous species over zeolites with different topologies[J]. J Fuel Chem Technol, 2018, 46(9):1101-1112. doi: 10.3969/j.issn.0253-2409.2018.09.009 [12] NIU X, GAO J, WANG K, MIAO Q, DONG M, WANG G, FAN W, QIN Z, WANG J. Influence of crystal size on the catalytic performance of H-ZSM-5 and Zn/H-ZSM-5 in the conversion of methanol to aromatics[J]. Fuel Process Technol, 2017, 157:99-107. doi: 10.1016/j.fuproc.2016.12.006 [13] LI J, LIU M, LI S, GUO X, SONG C. Influence of diffusion and acid properties on methane and propane selectivity in methanol-to-olefins reaction[J]. Ind Eng Chem Res, 2019, 58(5):1896-1905. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7342b4b8ca9c6ebea5bf93a028b1a5ea [14] SHANG Y, WANG W, ZHAI Y, SONG Y, ZHAO X, MA T, WEI J, GONG Y. Seed-fused ZSM-5 nanosheet as a superior MTP catalyst:Synergy of micro/mesopore and inter/external acidity[J]. Microporous Mesoporous Mater, 2019, 276:173-182. doi: 10.1016/j.micromeso.2018.09.038 [15] XING A, ZHANG N, YUAN D, LIU H, SANG Y, MIAO P, SUN Q, LUO M. Relationship between acidity, defective sites, and diffusion properties of nanosheet ZSM-5 and its catalytic performance in the methanol to propylene reaction[J]. Ind Eng Chem Res, 2019, 58(28):12506-12515. doi: 10.1021/acs.iecr.9b00325 [16] HAMBALI H U, JALIL A A, TRIWAHYONO S, JAMIAN S F, FATAH N A A, ABDULRASHEED A A, SIANG T J. Unique structure of fibrous ZSM-5 catalyst expedited prolonged hydrogen atom restoration for selective production of propylene from methanol[J]. Int J Hydrogen Energy, 2019. [17] LOSCH P, PINAR A B, WILLINGER M G, SOUKUP K, CHAVAN S, VINCENT B, PALE P, LOUIS B. H-ZSM-5 zeolite model crystals:Structure-diffusion-activity relationship in methanol-to-olefins catalysis[J]. J Catal, 2017, 345:11-23. doi: 10.1016/j.jcat.2016.11.005 [18] GAO M, LI H, YANG M, ZHOU J, YUAN X, TIAN P, YE M, LIU Z. A modeling study on reaction and diffusion in MTO process over SAPO-34 zeolites[J]. Chem Eng J, 2019, 377:119668. doi: 10.1016/j.cej.2018.08.054 [19] CARO J, BÜLOW M, SCHIRMER W, KÄRGER J, HEINK W, PFEIFER H, ŽDANOV S P. Microdynamics of methane, ethane and propane in ZSM-5 type zeolites[J]. J Chem Soc, Faraday Trans, 1985, 81(10):2541-2550. doi: 10.1039/f19858102541 [20] VAN-DEN-BEGIN N, REES L V C, CARO J, BVLOW M, HUNGER M, KÄRGER J. Diffusion of ethane in silicalite-1 by frequency response, sorption uptake and nuclear magnetic resonance techniques[J]. J Chem Soc, Faraday Trans, 1989, 85(6):1501-1509. doi: 10.1039/f19898501501 [21] NOWAK A K, DEN OUDEN C J J, PICKETT S D, SMIT B, CHEETHAM A K, POST M F M, THOMAS J M. Mobility of adsorbed species in zeolites:Methane, ethane, and propane diffusivities[J]. J Phys Chem, 1991, 95(2):848-854. doi: 10.1021/j100155a067 [22] JOBIC H, BÉE M, KEARLEY G J. Dynamics of ethane and propane in zeolite ZSM-5 studied by quasi-elastic neutron scattering[J]. Zeolites, 1992, 12(2):146-151. doi: 10.1016/0144-2449(92)90075-Z [23] SONG L, REES L V C. Frequency response diffusion of propane in silicalite-1[J]. Microporous Mater, 1996, 6(5/6):363-374. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ff5800e1c7ab6f8a02fadc266032e204 [24] SONG L, REES L V C. Diffusion of propane in theta-1 and silicalite-1 zeolites[J]. Microporous Mesoporous Mater, 2000, 41(1/3):193-200. http://cn.bing.com/academic/profile?id=f5ef5fa81aa7094d1ce2774509bafc10&encoded=0&v=paper_preview&mkt=zh-cn [25] 秦玉才, 高雄厚, 裴婷婷, 郑兰歌, 王琳, 莫周胜, 宋丽娟.噻吩在稀土离子改性Y型分子筛上吸附与催化转化研究[J].燃料化学学报, 2013, 41(7):889-896. doi: 10.3969/j.issn.0253-2409.2013.07.017QIN Yu-cai, GAO Xiong-hou, PEI Ting-ting, ZHENG Lan-ge, WANG Lin, MO Zhou-sheng, SONG Li-juan. Adsorption and catalytic conversion of thiophene on Y-type zeolites modified with rare-earth metal ions[J]. J Fuel Chem Technol, 2013, 41(7):889-896. doi: 10.3969/j.issn.0253-2409.2013.07.017 [26] 贾未鸣, 秦玉才, 张乐, 莫周胜, 宋丽娟, 孙兆林. Ce改性对Y型分子筛酸中心可接近性及催化活性的影响[J].石油炼制与化工, 2017, 48(6):14-19. doi: 10.3969/j.issn.1005-2399.2017.06.005JIA Wei-ming, QIN Yu-cai, ZHANG Le, MO Zhou-sheng, SONG Li-juan, SUN Zhao-lin. Study on accessibility and catalytic activity of Y zeolite modified by Ce-species[J]. Pet Process Petrochem, 2017, 48(6):14-19. doi: 10.3969/j.issn.1005-2399.2017.06.005 [27] SONG L, REES L V C. Adsorption and diffusion of cyclic hydrocarbon in MFI-type zeolites studied by gravimetric and frequency-response techniques[J]. Microporous Mesoporous Mater, 2000, 35:301-314. http://cn.bing.com/academic/profile?id=9d8d380967d5fa8a4a61fa1ecd8daad3&encoded=0&v=paper_preview&mkt=zh-cn [28] REES L V C, SONG L. Frequency response method for the characterisation of microporous solids[J]. Membrane Sci Technol, 2000, 6(3):139-186. http://cn.bing.com/academic/profile?id=48ece5bcb9c130384b27a521e67ad002&encoded=0&v=paper_preview&mkt=zh-cn [29] YASUDA Y, SAEKI M. Kinetic details of a gas-surface system by the frequency response method[J]. J Phys Chem, 1978, 82(1):74-80. doi: 10.1021/j100490a019 [30] REES L V C, SONG L. Recent Advances in Gas Separation by Microporous Ceramic Membranes[M]. Amsterdam:Membrane Science and Technology, Elsevier, 2000, 39-186. [31] SONG L, SUN Z L, REES L V C. 19-O-02-Studies of adsorption, diffusion and molecular simulation of cyclic hydrocarbons in MFI zeolites[J]. Stud Surf Sci Catal, 2001, 135:153. http://www.sciencedirect.com/science/article/pii/S0167299101812556 [32] YASUDA Y, SUZUKI Y, FUKADA H. Kinetic details of a gas/porous adsorbent system by the frequency response method[J]. J Phys Chem, 1991, 95(6):2486-2492. doi: 10.1021/j100159a070 [33] YASUDA Y. Detection of surface resistance in a gas/porous-adsorbent system by frequency response method[J]. Bull Chem Soc Jpn, 1991, 64(3):954-961. doi: 10.1246/bcsj.64.954 [34] YASUDA Y. Frequency-response method for investigation of gas-surface dynamic phenomena[J]. Heterogen Chem Rev, 1994, 1(2):103-124. [35] SHEN D, REES L V C. Frequency response study of single-file diffusion in theta-1[J]. J Chem Soc, Faraday Trans, 1994, 90(19):3017. doi: 10.1039/ft9949003017 [36] CAI D, WANG N, CHEN X, MA Y, HOU Y, LI X, ZHANG C, CHEN Z, SONG W, ARSLAN M T, LI Y, WANG Y, QIAN W, WEI F. Highly selective conversion of methanol to propylene:Design of an MFI zeolite with selective blockage of (010) surfaces[J]. Nanoscale, 2019, 11(17):8096-8101. doi: 10.1039/C8NR10371B [37] ZHANG W, CHN J, XU S, CHU Y, WEI Y, ZHI Y, HUANG J, ZHENG A, WU X, MENG X, XIAO F, DENG F, LIU Z. Methanol to olefins reaction over cavity-type zeolite:Cavity controls the critical intermediates and product selectivity[J]. ACS Catal, 2018, 8(12):10950-10963. doi: 10.1021/acscatal.8b02164 -





 下载:
下载: