-
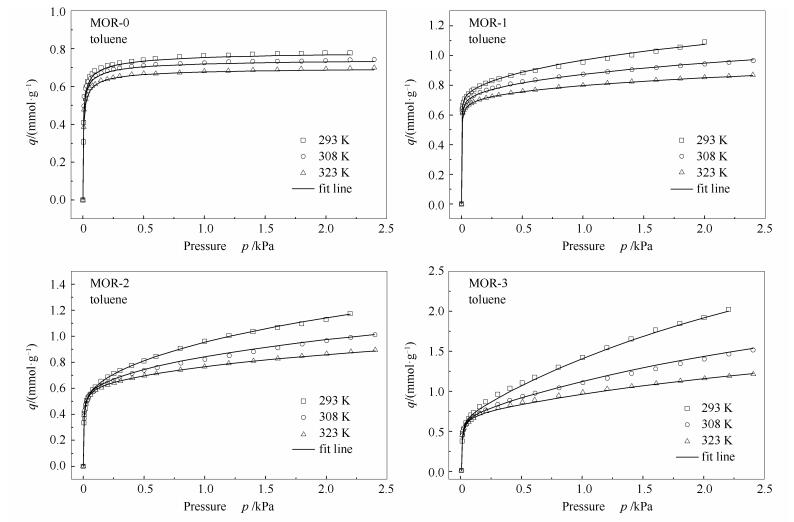
摘要: 为了考察多级孔丝光沸石中介孔的存在对丝光沸石吸附平衡和动力学的影响,选择甲苯分子作为探针分子,对其在具有不同介孔孔隙度的多级孔丝光沸石上的吸附等温线和吸附动力学曲线进行了测试。结果表明,甲苯在多级孔丝光沸石上的吸附等温线可以很好地用双位Toth吸附模型进行描述,由拟合参数以及亨利常数(KH)和初始吸附热(Qst)的计算得知,相对于微孔丝光沸石,介孔的引入增大了甲苯在丝光沸石内的吸附量,但减弱了甲苯与沸石表面的相互作用力;另外,甲苯在多级孔沸石表现出高的吸附速率,并随介孔孔隙度的增加而增大,反映了沸石内介孔的存在可有效促进沸石的传质能力。
-
关键词:
- 多级孔丝光沸石 /
- 甲苯 /
- 吸附等温线 /
- 吸附动力学 /
- 双位Toth吸附模型
Abstract: The adsorption isotherms and kinetic curves of toluene on a series of hierarchical mordenite zeolites with different mesoporosities were measured to investigate the effect of hierarchical pore structures of mordenite on the adsorption and kinetics. The isotherms of hierarchical mordenites show the combination of characteristics of both micropore and mesopore adsorption. Furthermore, the fitting of experimental isothermal data of toluene reveals that the isotherms of toluene can be well described by dual-sites Toth-type model. The fitting parameters and the Henry's constants (KH) and the initial heats of adsorption (Qst) calculated show that the introduction of mesopores into mordenite weakens the interaction between toluene and zeolitic surface. Additionally, the adsorption kinetic curves show that the adsorption rates of toluene on hierarchical mordenite are much larger than that on microporous mordenite, revealing the enhanced effect of mesopore on the mass transfer in zeolites. -
表 1 甲苯在丝光沸石上的吸附等温线双位Toth模型拟合参数
Table 1 Dual-site Toth model fitting parameters of toluene on mordenites
Parameter MOR-0 MOR-1 MOR-2 MOR-3 qs1, 0 /(mmol·g-1) 0.74 0.70 0.68 0.66 χ1 1.41 0.68 0.13 0.56 b1, 0/(kPa-1) 1230 1070 840 760 Q1/(kJ·mol-1) 15.87 12.01 9.52 8.09 t1, 0 0.53 0.88 0.46 0.51 α1 1.71 0.88 0.79 1.31 qs2, 0/(mmol·g-1) 0.02 1.11 1.67 1.92 χ2 0.01 0.31 4.85 7.98 b2, 0/(kPa-1) 1.5 0.7 0.4 0.3 Q2/(kJ·mol-1) 13.71 12.82 7.41 4.67 t2, 0 0.68 0.46 0.63 1.37 α2 0.65 0.10 0.01 0.24 表 2 甲苯在丝光沸石上的亨利常数和初始吸附热
Table 2 Henry's constants and initial heats of adsorption for toluene on mordenite samples
Sample T/K KH/(mmol·g-1·kPa-1) Qst/(kJ·mol-1) MOR-0 293 4.02×105 82.7 308 1.46×105 323 5.96×104 MOR-1 293 2.99×104 67.8 308 1.62×104 323 6.60×103 MOR-2 293 4.16×103 57.4 308 2.28×103 323 1.25×103 MOR-3 293 1.64×103 46.4 308 0.86×103 323 0.61×103 -
[1] VOS A M, ROZANSKA X, SCHOONHEYDT R A, VAN SANTEN R A, HUTSCHKA F, HAFNER J. A theoretical study of the alkylation reaction of toluene with methanol catalyzed by acidic mordenite[J]. J Am Chem Soc, 2001, 123(12):2799-2809. doi: 10.1021/ja001981i [2] PEREGO C, INGALLINA P. Combining alkylation and transalkylation for alkylaromatic production[J]. Green Chem, 2004, 6(6):274-279. doi: 10.1039/b403277m [3] KORTUNOV P, VASENKOV S, KÄRGER J, VALIULLIN R, GOTTSCHALK P, FÉELÍA M, PEREZ M, STÖCKER M, DRESCHER B, MCELHINEY G, BERGER C, GLÄSER R, WEITKAMP J. The role of mesopores in intracrystalline transport in USY zeolite:PFG NMR diffusion study on various length scales[J]. J Am Chem Soc, 2005, 127(37):13055-13059. doi: 10.1021/ja053134r [4] MOLINER M, ROMAN-LESHKOV Y, DAVIS M E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water[J]. Proc Natl Acad Sci USA, 2010, 107(14):6164-6168. doi: 10.1073/pnas.1002358107 [5] MARCILLY C R. Where and how shape selectivity of molecular sieves operates in refining and petrochemistry catalytic processes[J]. Top Catal, 2000, 13(4):357-366. doi: 10.1023/A:1009007021975 [6] CHEN N Y, DEGNAN T F, SMITH C M. Molecular transport and reaction in zeolites:Design and application of shape selective catalysts[J]. Z Phys Chem, 1995, 191(Part_2):282. http://www.wiley.com/WileyCDA/WileyTitle/productCd-0471185485.html [7] RUTHVEN D M. Diffusion in zeolites and other microporous solids[J]. Z Phys Chem, 1992, 92(Part_2):269-270. [8] GUISNET M, MAGNOUX P. Coking and deactivation of zeolites:Influence of the Pore Structure[J]. Appl Catal, 1989, 54(1):1-27. doi: 10.1016/S0166-9834(00)82350-7 [9] PEREZ R J, CHRISTENSEN C H, EGEBLAD K, CHRISTENSEN C H, GROEN J C. Enhanced utilization of microporous crystals in catalysis by advances in materials design[J]. Chem Soc Rev, 2008, 37(11):2530-2542. doi: 10.1039/b809030k [10] OLSBYE U, SVELLE S, BJØRGEN M, BEATO P, JANSSENS T V W, JOENSEN F, BORDIGA S, LILLERUD K P. Conversion of methanol to hydrocarbons:How zeolite cavity and pore size controls product selectivity[J]. Angew Chem, 2012, 51(24):5810-5831. doi: 10.1002/anie.201103657 [11] GROEN J C, ZHU W, BROUWER S, HUYNINK S J, KAPTEIJN F, MOULIJN A J A, PÉREZRAMÍREZ J. Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication[J]. J Am Chem Soc, 2007, 129(2):355-360. doi: 10.1021/ja065737o [12] JANSSEN A H. Generation, characterization, and impact of mesopores in zeolite catalysts[J]. Catal Rev, 2003, 45(2):297-319. doi: 10.1081/CR-120023908 [13] KIM J, CHOI M, RYOO R. Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process[J]. J Catal, 2010, 269(1):219-228. doi: 10.1016/j.jcat.2009.11.009 [14] HARTMANN M. Hierarchical zeolites:A proven strategy to combine shape selectivity with efficient mass transport[J]. Cheminform, 2005, 36(3):5880-5882. [15] 王海彦, 宋盼盼, 王钰佳.多级孔Hβ沸石对NiWP/Hβ-Al2O3催化剂柴油加氢性能的影响[J].燃料化学学报, 2016, 44(4):470-476. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18815.shtmlWANG Hai-yan, SONG Pan-pan, WANG Yu-jia. Influence of hierarchically mesoporous Hβ zeolite on the performance of NiWP/Hβ-Al2O3 catalysts in diesel oil hydro-upgrading[J]. J Fuel Chem Technol, 2016, 44(4):470-476. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18815.shtml [16] BAERLOCHER C, MEIER W M, OLSON D H. Atlas of Zeolite Framework Types[M]. Hodder and Stoughton:Elsevier, 1922. [17] VAN DONK S, BROERSMA A, GIJZEMAN O L J, VAN BOKHOVEN J A, BITTER J H, DE JONG K P. Combined diffusion, adsorption, and reaction studies of n-hexane hydroisomerization over Pt/H-mordenite in an oscillating microbalance[J]. J Catal, 2001, 204(2):272-280. doi: 10.1006/jcat.2001.3393 [18] SCHMITZ A D, SONG C. Shape-selective isopropylation of naphthalene. Reactivity of 2, 6-diisopropylnaphthalene on dealuminated mordenites[J]. Catal Today, 1996, 31(1):19-25. [19] LEI G D, CARVILL B T, SACHTLER W M H. Single file diffusion in mordenite channels:Neopentane conversion and H/D exchange as catalytic probes[J]. Appl Catal A:Gen, 1996, 142(2):347-359. doi: 10.1016/0926-860X(96)00062-2 [20] DEJAIFVE P, AUROUX A, GRAVELLE P C, VÉDRINE J C, GABELICA Z, DEROUANE E G. Methanol conversion on acidic ZSM-5, offretite, and mordenite zeolites:A comparative study of the formation and stability of coke deposits[J]. J Catal, 1981, 70(1):123-136. doi: 10.1016/0021-9517(81)90322-5 [21] GROEN J C, SANO T, MOULIJN J A, PÉREZ-RAMÍREZ J. Alkaline-mediated mesoporous mordenite zeolites for acid-catalyzed conversions[J]. J Catal, 2007, 251(1):21-27. doi: 10.1016/j.jcat.2007.07.020 [22] SAXENA S K, VISWANADHAM N. Enhanced catalytic properties of mesoporous mordenite for benzylation of benzene with benzyl alcohol[J]. Appl Surf Sci, 2017, 392:384-390. doi: 10.1016/j.apsusc.2016.09.062 [23] ORDOMSKY V V, IVANOVA I I, KNYAZEVA E E, YUSCHENKO V V, ZAIKOVSKⅡ V I. Cumene disproportionation over micro/mesoporous catalysts obtained by recrystallization of mordenite[J]. J Catal, 2012, 295(11):207-216. [24] DAVIS M E, DAVIS R J. Fundamentals of Chemical Reaction Engineering[M]. New York:McGraw-Hill Higher Education, 2012. [25] VOS A M, ROZANSKA X, SCHOONHEYDT R A, SANTEN R A V, HUTSCHKA F, HAFNER J. A theoretical study of the alkylation reaction of toluene with methanol catalyzed by acidic mordenite[J]. J Am Chem Soc, 2001, 123(12):2799-2809. doi: 10.1021/ja001981i [26] OLSON D H, HAAG W O. Structure-Selectivity Relationship in Xylene Isomerization and Selective Toluene Disproportionation[M]. New York:ACS Publications, 1984. [27] ČEJKA J, WICHTERLOVA B. Acid-catalyzed synthesis of mono-and dialkyl benzenes over zeolites:Active sites, zeolite topology, and reaction mechanisms[J]. Catal Rev, 2002, 44(3):375-421. [28] DAI G, HAO W M, XIAO H, MA J H, LI R F. Hierarchical mordenite zeolite nano-rods bundles favourable to bulky molecules[J]. Chem Phys Lett, 2017, 686(31):111-115. [29] SONG A, MA J, XU D, LI R. Adsorption and diffusion of xylene Isomers on mesoporous beta zeolite[J]. Catalysts, 2015, 5(4):2098-2114. doi: 10.3390/catal5042098 [30] WEISS R F. Carbon dioxide in water and seawater:The solubility of a non-ideal gas[J]. Mar Chem, 1974, 2(3):203-215. doi: 10.1016/0304-4203(74)90015-2 [31] ZHAO H, MA J H, ZHANG Q Q, LIU Z P, LI R F. Adsorption and diffusion of n-heptane and toluene over mesoporous ZSM-5 zeolites[J]. Ind Eng Chem Res, 2014, 53(35):13810-13819. doi: 10.1021/ie502496v [32] XU D, MA J H, SONG A X, LIU Z P, LI R F. Availability and interconnectivity of pores in mesostructured ZSM-5 zeolites by the adsorption and diffusion of mesitylene[J]. Adsorption, 2016, 22(8):1083-1090. doi: 10.1007/s10450-016-9830-9 -





 下载:
下载: