Preparation of NiWO4/g-C3N4 and its ultra-deep desulfurization properties in ionic liquid
-
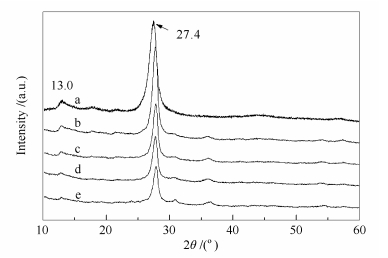
摘要: 采用水热法合成了NiWO4纳米粒子,然后通过混合煅烧法成功地制备了负载型催化剂NiWO4/g-C3N4。采用XRD、FT-IR、EDS、SEM、BET和XPS表征了NiWO4/g-C3N4的形貌和结构特征。以NiWO4/g-C3N4为催化剂,过氧化氢为氧化剂,1-丁基-3-甲基咪唑四氟硼酸盐离子液体([BMIM]BF4)为萃取剂。考察了催化剂的负载量,过氧化氢、离子液体和催化剂使用量,反应温度,反应时间,不同种类的含硫化合物对脱硫效果的影响。结果表明,在5 mL模拟油,0.2 mL过氧化氢,1.0 mL的[BMIM]BF4,0.03 g的NiWO4/g-C3N4,反应温度为80 ℃,反应时间为140 min的最佳的反应条件下,脱硫率可以达到97.35%。实验表明,NiWO4/g-C3N4具有很好的催化稳定性,催化剂重复使用五次后催化活性并没有明显地降低。
-
关键词:
- NiWO4/g-C3N4 /
- 氧化脱硫 /
- 二苯并噻吩 /
- 离子液体
Abstract: The NiWO4 nanoparticles were synthesized by hydrothermal method. The supported catalysts NiWO4/g-C3N4 were prepared by a simple mixing-calcination method. XRD, FT-IR, EDS, SEM, BET and XPS were used to characterize the morphology and structure of NiWO4/g-C3N4. The prepared NiWO4/g-C3N4 was used as catalyst, hydrogen peroxide as oxidant, 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid ([BMIM]BF4) as extractant for oxidative desulfurization. The effects of catalyst loading, the amount of hydrogen peroxide, ionic liquid and catalyst, reaction temperature, reaction time, and different sulfur compounds on desulfurization efficiency were studied. The desulfurization rate can reach 97.35% at the optimum reaction conditions:5 mL of model oil, 0.2 mL of hydrogen peroxide, 1.0 mL of [BMIM] BF4, 0.03 g of NiWO4/g-C3N4, 80 ℃ of reaction temperature and 140 min of reaction time. The results showed that NiWO4/g-C3N4 had good catalytic stability, and the catalytic activity was not significantly reduced after 5 repeated reactions.-
Key words:
- NiWO4/g-C3N4 /
- oxidative desulfurization /
- dibenzothiophene /
- ionic liquid
-
表 1 不同负载量的NiWO4/g-C3N4催化剂的比表面积
Table 1 Specific surface area of g-C3N4, NiWO4 and NiWO4/g-C3N4 with different loading amount
Catalyst Specific surface area A/(m2·g-1) g-C3N4 18.15 NiWO4 112.60 5%-NiWO4/g-C3N4 40.12 10%-NiWO4/g-C3N4 43.60 15%-NiWO4/g-C3N4 46.76 20%-NiWO4/g-C3N4 48.58 表 2 20%-NiWO4/g-C3N4循环次数使用对脱硫效果的影响
Table 2 Influence of the recycle times of 20%-NiWO4/g-C3N4 on the desulfurization rate
Number of recycle 1 2 3 4 5 Desulfurization rate η /% 97.35 96.33 96.18 95.87 95.47 -
[1] LORENÇON E, ALVES D C B, KRAMBROCK K, ERICK S Á, RESENDE R R, FERLAUTO A S, LAGO R M. Oxidative desulfurization of dibenzothiophene over titanate nanotubes[J]. Fuel, 2014, 132:53-61. doi: 10.1016/j.fuel.2014.04.020 [2] YAN X M, SU G S, XIONG L. Oxidative desulfurization of diesel oil over Ag-modified mesoporous HPW/SiO2catalyst[J]. J Fuel Chem Technol, 2009, 7(3):318-323. http://en.cnki.com.cn/Article_en/CJFDTOTAL-RLHX200903015.htm [3] BAZYARI A, KHODADADI A A, MAMAGHANI A H, BEHESHTIAN J, THOMPSON L T, MORTAZAVI Y. Microporous titania-silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization[J]. Appl Catal B:Environ, 2016, 180:65-77. doi: 10.1016/j.apcatb.2015.06.011 [4] STANISLAUS A, MARAFI A, RANA M S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production[J].Catal Today, 2010, 153(1):1-68. http://www.sciencedirect.com/science/article/pii/S0920586110003299 [5] JIANG B, YANG H, ZHANG L, ZHANG R Y, SUN Y L, HUANG Y. Efficient oxidative desulfurization of diesel fuel using amide-based ionic liquids[J]. Chem Eng J, 2016, 283:89-96. doi: 10.1016/j.cej.2015.07.070 [6] LI C, LI D, ZOU S, LI Z, YIN J M, WANG A L, CUI Y N, YAO Z L, ZHAO Q. Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents[J].Green Chem, 2013, 15(10):2793-2799. doi: 10.1039/c3gc41067f [7] HUANG L, WANG G, QIN Z, DONG M, DU M X, GE H, LI X K, ZHAO Y D, ZHANG J, HU T D, WANG J G. In situ XAS study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO[J]. Appl Catal B:Environ, 2011, 106(1):26-38. http://www.sciencedirect.com/science/article/pii/S0926337311002025 [8] MARGETA D, SERTIĆ-BIONDA K, FOGLAR L. Ultrasound assisted oxidative desulfurization of model diesel fuel[J]. Appl Acoust, 2016, 103(2):202-206. http://www.sciencedirect.com/science/article/pii/S0003682X15001905 [9] WU L, SITAMRAJU S, XIAO J, LIU B, LI Z, JANIK M J, SONG C S. Effect of liquid-phase O3 oxidation of activated carbon on the adsorption of thiophene[J]. Chem Eng J, 2014, 242:211-219. doi: 10.1016/j.cej.2013.12.077 [10] BAKAR W A W A, ALI R, KADIR A A A, MOKHTAR W N A W. Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel[J]. Fuel Process Technol, 2012, 101:78-84. doi: 10.1016/j.fuproc.2012.04.004 [11] GARCÍA-GUTIÉRREZ J L, LAREDO G C, GARCÍA-GUTIÉRREZ P, JIMÉNEZ-CRUZ F. Oxidative desulfurization of diesel using promising heterogeneous tungsten catalysts and hydrogen peroxide[J]. Fuel, 2014, 138:118-125. doi: 10.1016/j.fuel.2014.07.049 [13] SHI X Y, SUN M, FAN J, WANG P M, MA W J, WEI J F. Deep oxidative desulfurization of benzothiophene and dibenzothiophene with a peroxophosphotungstate-ionic liquid brush assembly[J].Appl Organomet Chem, 2015, 29(9):633-637. doi: 10.1002/aoc.v29.9 [14] ZHU W, WU P, YANG L, CHANG Y H, CHAO Y H, LI H M, JIANG Y Q, JIANG W, XUN S H. Pyridinium-based temperature-responsive magnetic ionic liquid for oxidative desulfurization of fuels[J]. Chem Eng J, 2013, 229(4):250-256. http://www.sciencedirect.com/science/article/pii/S1385894713007572 [15] WAN M W, YEN T F. Enhance efficiency of tetraoctylammonium fluoride applied to ultrasound-assisted oxidative desulfurization (UAOD) process[J]. Appl Catal A:Gen, 2007, 319(1):237-245. http://www.sciencedirect.com/science/article/pii/S0926860X06008854 [16] ZHANG M, ZHU W, XUN S, LI H M, GU Q Q, ZHAO Z, WANG Q. Deep oxidative desulfurization of dibenzothiophene with POM-based hybrid materials in ionic liquids[J]. Chem Eng J, 2013, 220(6):328-336. http://www.sciencedirect.com/science/article/pii/S138589471300096X [17] SHAN J H, CHEN L, SUN L B, LIU X Q. Adsorptive removal of thiophene by Cu-modified mesoporous silica MCM-48 derived from direct synthesis[J]. Energy Fuels, 2011, 25(7):3093-3099. doi: 10.1021/ef200472j [18] ZHU W, XU Y, LI H, DAI B L, XU H, WANG CHAO, CHAO Y H, LIU H. Photocatalytic oxidative desulfurization of dibenzothiophene catalyzed by amorphous TiO2, in ionic liquid[J]. Korean J Chem Eng, 2014, 31(2):211-217. doi: 10.1007/s11814-013-0224-3 [19] SUN B, ZHAO W, WEI L, LI H W, CHEN P. Enhanced resistive switching effect upon illumination in self-assembled NiWO4 nano-nests[J]. Chem Commun, 2014, 50(86):13142-5. doi: 10.1039/C4CC05784H [20] LIU D, GUI J, PENG X, YANG S, SUN Z L. Deep oxidative desulfurization of real diesel catalyzed by Na2WO4, in ionic liquid[J]. Energ Source Part A, 2013, 35(1):1-8. doi: 10.1080/15567036.2010.503230 [21] 邢鹏飞, 石薇薇, 李秀萍, 赵荣祥. NiWO4的制备及其在离子液体中深度氧化脱硫的应用[J].石油学报(石油加工), 2017, 33(2):334-342. http://www.cqvip.com/QK/94167X/201702/671649453.htmlXING Peng-fei, SHI Wei-wei, LI Xiu-ping, ZHAO Rong-xiang.Preparation of NiWO4 catalyst and its application in the oxidationde sulfurization of model oil[J]. Acta Pet Sin (Pet Process Sect) 2017, 33(2):334-342. http://www.cqvip.com/QK/94167X/201702/671649453.html [22] XING P F, ZHAO R X, LI X P, GAO X H. Preparation of CoWO4/g-C3N4and its ultra-deep desulfurization property[J]. Aust J Chem, ,70(3):271-279. doi: 10.1071/CH16320 [23] WANG X C, MAEDA K, THOMAS A, TAKANABE K, XIN G, CARLSSON J M, DOMEN K, ANTONIETTI M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nat Mater, 2009, 8(1):76-80. doi: 10.1038/nmat2317 [24] JING D, LIU Q, ZHANG Z, LIU X, ZHAO J Q, CHENG S B, ZONG B N, DAI W L. Carbon nitride nanosheets decorated with WO3, nanorods:Ultrasonic-assisted facile synthesis and catalytic application in the green manufacture of dialdehydes[J]. Appl Catal B:Environ, 2015, 165:511-518. doi: 10.1016/j.apcatb.2014.10.037 [25] YAN H, CHEN Y, XU S. Synthesis of graphitic carbon nitride by directly heating sulfuric acid treated melamine for enhanced photocatalytic H2 production from water under visible light[J].Int J Hydrogen Energy, 2012, 37(1):125-133. doi: 10.1016/j.ijhydene.2011.09.072 [26] 宋继梅, 刘晓灵, 董纳, 李文慧, 司维, 杨捷.介孔板栗状NiWO4吸附剂的制备及其性能[J].安徽大学学报(自科版), 2016, 40(1):73-79. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=ahdx201601012&dbname=CJFD&dbcode=CJFQSONG Ji-mei, LIU Xiao-ling, DONG Na, LI Wen-hui, SI Wei, YANG Jie.Preparation and properties of Mesoporous NiWO4 nanospheres[J]. J Anhui Univ(Nat Sci), 2016, 40(1):73-79. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=ahdx201601012&dbname=CJFD&dbcode=CJFQ [27] GREEN S V, KUZMIN A, PURANS J, GRANQVIST C G, NIKLASSON G A. Structure and composition of sputter-deposited nickel-tungsten oxide films[J]. Thin Solid Films, 2011, 519(7):2062-2066. doi: 10.1016/j.tsf.2010.10.033 [28] GU Y, CHEN L, SHI L, MA J H, YANG Z, QIAN Y T. Synthesis of C3N4 and graphite by reacting cyanuric chloride with calcium cyanamide[J]. Carbon, 2003, 41(13):2674-2676. doi: 10.1016/S0008-6223(03)00357-9 [29] TALAPANENI S N, ANANDAN S, MANE G P, ANAND C, DHAWALE D S, VARGHESE S, MANO A, MORI T, VINU A. Facile synthesis and basic catalytic application of 3D mesoporous carbon nitride with a controllable bimodal distribution[J]. J Mater Chem, 2012, 22(19):9831-9840. doi: 10.1039/c2jm30229b [30] DONG F, WU L, SUN Y, FU M, WU Z B, LEE S C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts[J]. J Mater Chem, 2011, 21(39):15171-15174 doi: 10.1039/c1jm12844b [31] NG K T, HERCULES D M. Studies of nickel-tungsten-alumina catalysts by X-ray photoelectron spectroscopy[J]. J Phys Chem, 1976, 80(19):2094-2102. doi: 10.1021/j100560a009 [32] MOULDER J F, STICKLE W F, SOBOL P E, BOMBEN K D, CHASTAIN J. Handbook of X-ray photoelectron spectroscop[J]. Chem Phys Lett, 1979, 220(1):7-10. https://www.researchgate.net/publication/284949135_Handbook_of_X-Ray_Photoelectron_Spectroscop [33] MANCHEVA M N, IORDANOVA R S, KLISSURSKI DIMITAR G, TYULIEV G T, KUNEV B N. Direct mechanochemical synthesis of nanocrystalline NiWO4[J]. J Phys Chem C, 2007, 111(3):1101-1104. doi: 10.1021/jp065071k [34] ZHANG J, ZHU W, LI H, JIANG W, JIANG Y Q, HUANG W L, YAN Y S. Deep oxidative desulfurization of fuels by Fenton-like reagent in ionic liquids[J]. Green Chem, 2009, 11(11):1801-1807. doi: 10.1039/b914130h [35] LI L, ZHANG J, SHEN C, WANG Y J, LUO G S. Oxidative desulfurization of model fuels with pure nano-TiO2 as catalyst directly without UV irradiation[J]. Fuel, 2015, 167:9-16. https://www.researchgate.net/publication/284358449_Oxidative_desulfurization_of_model_fuels_with_pure_nano-TiO2_as_catalyst_directly_without_UV_irradiation [36] LÜ H, REN W, WANG H, WANG Y, CHEN W, SUO Z H. Deep desulfurization of diesel by ionic liquid extraction coupled with catalytic oxidation using an Anderson-type catalyst[(C4H9)4 N]4NiMo6O24H6[J]. Appl Catal A:Gen, 2013, 453:376-382. doi: 10.1016/j.apcata.2012.12.047 [37] LÜ H, DENG C, REN W, YANG X. Oxidative desulfurization of model diesel using[(C4H9)4N]6Mo7O24, as a catalyst in ionic liquids[J]. Fuel Process Technol, 2014, 119(1):87-91. [38] 李宇慧, 冯丽娟, 王景刚, 周旋, 程斌斌, 王晓燕, 李春虎. MoO3/介孔Al2O3催化氧化脱除模拟油中的硫[J].高等学校化学学报, 2011, 32(3):778-782. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gdxh201103059&dbname=CJFD&dbcode=CJFQLI Yu-hui, FENG Li-juan, WANG Jing-gang, ZHOU Xuan, CHENG Bin-bin, WANG Xiao-yan, LI Chun-hu. Catalytic oxidative desulfurization of model oil by MoO3/mesoporous Al2O3[J]. Chem J Chin Univ, 2011, 32(3):778-782. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gdxh201103059&dbname=CJFD&dbcode=CJFQ [39] 苏建勋, 艾东, 赵荣祥, 李秀萍. CuWO4/C复合物的制备和其在模拟油氧化脱硫中的应用[J].燃料化学学报, 2015, 43(12):1476-1481. doi: 10.3969/j.issn.0253-2409.2015.12.011SU Jian-xun, AI Dong, ZHAO Rong-xiang, LI Xiu-ping. Study of the preparation of CuWO4/C composite and it's application in oxidative desulfurization of model oil[J]. J Fuel Chem Technol, 2015, 43(12):1476-1481. doi: 10.3969/j.issn.0253-2409.2015.12.011 [40] YI N, DONG Y, LU B, DONG H F, ZHANG X P. Fast oxidative desulfurization of fuel oil using dialkylpyridinium tetrachloroferrates ionic liquids[J]. Fuel, 2013, 103(1):997-1002. http://www.sciencedirect.com/science/article/pii/S0016236112006321 [41] OTSUKI S, NONAKA T, TAKASHIMA N, QIAN W H, ISHIHARA A, IMAI T, KABE T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction[J]. Energy Fuels, 2000, 14(6):1232-1239. doi: 10.1021/ef000096i [42] LÜ H Y, GAO J B, JIANG Z X, FEI J, YANG Y X, WANG G, LI C. Ultra-deep desulfurization of diesel by selective oxidation with[C18H37N(CH3)3]4[H2NaPW10O36] catalyst assembled in emulsion droplets[J]. J Catal, 2006, 239:369-375. doi: 10.1016/j.jcat.2006.01.025 [43] 李佳慧, 胡嘉, 赵荣祥, 李秀萍.氨基酸功能化磷钨酸盐的制备及其催化氧化脱硫性能[J].燃料化学学报, 2014, 42(11):1394-1399. doi: 10.3969/j.issn.0253-2409.2014.11.018LI Jia-hui, HU Jia, ZHAO Rong-xiang, LI Xiu-ping. Prepartion of amino acid functionalized heteropolyacid salt and its catalytic performance for oxidation desulfurization of model oil[J]. J Fuel Chem Technol, 2014, 42(11):1394-1399. doi: 10.3969/j.issn.0253-2409.2014.11.018 [44] ABDALLA Z E A, LI B, TUFAIL A. Preparation of phosphate promoted Na2WO4/Al2O3 catalyst and its application for oxidative desulfurization[J]. J Ind Eng Chem, 2009, 15(6):780-783. doi: 10.1016/j.jiec.2009.09.026 [45] USUI Y, SATO K A. Green method of adipic acid synthesis:organic solvent-and halide-free oxidation of cycloalkanones with 30% hydrogen peroxide[J]. Green Chem, 2003, 5(4):373-375. doi: 10.1039/b305847f [46] EDE S R, KUNDU S. Microwave synthesis of SnWO4 nanoassemblies on DNA scaffold:A novel material for high performance supercapacitor and as catalyst for butanol oxidation[J]. ACS Sustainable Chem Eng, 2015, 3(9):2321-2336. doi: 10.1021/acssuschemeng.5b00627 -





 下载:
下载: