Comparative study of K2CO3 and Na2CO3 in the process of coal gangue catalytic gasification coupled with aluminum extraction from gasification ash
-
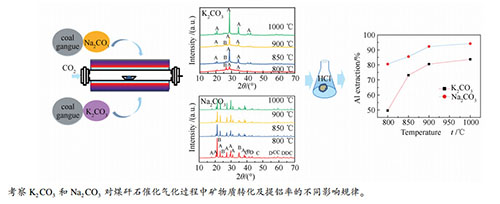
摘要: 以煤矸石为研究对象,对比研究了Na2CO3与K2CO3对煤矸石催化气化反应性及催化气化灰中Al的溶出行为的影响。同时,采用X射线衍射分析(XRD)和热重分析(TGA)研究了不同催化剂及温度作用下矸石中矿物质的热转变过程。结果表明,与K2CO3相比,煤矸石中的高岭石更容易与Na2CO3反应生成钠霞石,而酸浸可实现钠霞石中铝和硅元素的有效分离。此外,Na2CO3作为催化剂时,所得气化灰经盐酸浸取后铝的浸出率可达到94.2%。而K2CO3作催化剂时,其铝的浸出率只有83.7%。因此,对矸石催化气化耦合气化灰的铝提取来说,Na2CO3催化剂具有更好的选择性。Abstract: Coal gangue as the research object of this study, the effects of Na2CO3 and K2CO3 on the gasification reactivity and the dissolution behavior of Al from catalytic gasification were compared. At the same time, X-ray diffraction (XRD) and thermogravimetric analysis (TGA) were used to analyze the thermal-conversion process of mineral in coal gangue with different catalysts and at different temperatures. The results show that compared with K2CO3, Na2CO3 can react more easily with the kaolinite in coal gangue to form nepheline, which can achieve effective separation of aluminum and silicon by acid leaching. Moreover, using Na2CO3 as catalyst, the Al extraction rate of gasification ash treated by hydrochloric acid can reach 92.3%, while it can only reach 83.7% using K2CO3 as catalyst. Therefore, Na2CO3 has better selectivity for the coal gangue catalytic gasification coupled with aluminum extraction from gasification ash.
-
Key words:
- aluminum leaching rate /
- Na2CO3 /
- K2CO3 /
- minerals transformation
-
表 1 原煤矸石的工业分析和元素分析
Table 1 Proximate and ultimate analyses of coal gangue sample
Proximate analysis wad/% Ultimate analysis wdaf/% M A V FC C H Oa N S 0.93 65.00 17.07 17.00 62.46 6.23 30.14 0.88 0.29 a: by difference 表 2 灰成分分析
Table 2 Ash compositions of coal gangue sample
Sample Content w/% SiO2 Al2O3 Fe2O3 CaO MgO P2O5 TiO2 K2O Na2O Cl2O XCG 52.51 44.96 0.32 0.20 0.21 0.42 1.02 0.13 0.00 0.23 CGA-Na 47.01 33.42 0.63 0.13 0.12 0.03 0.81 0.21 17.54 0.10 CGA-K 39.11 32.19 0.66 0.15 0.08 0.37 0.92 26.4 0.00 0.12 表 3 800 ℃下气化灰和酸溶灰的能谱分析
Table 3 EDS analysis results of gasification ash and acid soluble ash at 800 ℃
Sample EDS analysis results w/% Si Al O K Na CGA-K 9.54 8.99 70.24 11.24 0.00 HCl-CGA-K 13.37 3.89 82.74 0.00 0.00 CGA-Na 9.47 7.39 73.06 0.00 10.08 HCl-CGA-Na 34.79 0.00 65.21 0.00 0.00 -
[1] BIAN Z F, DONG J H, LEI S G, LENG H L, MU S G, WANG H. The impact of disposal and treatment of coal mining wastes on environment and farmland[J]. Environ Geol, 2009, 58(3):625-634. doi: 10.1007/s00254-008-1537-0 [2] LI H, ZHENG F, WANG J, ZHOU J, HUANG X H, CHEN L, HU P, GAO J M, ZHEN Q, BASHIR S, LIU J H. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance[J]. Chem Eng J, 2020, 390:1-11. http://www.sciencedirect.com/science/article/pii/S1385894720305040 [3] CHENG F, CUI L, MILLER J D, WANG, X. Aluminum leaching from calcined coal waste using hydrochloride ORIC acid solution[J]. Miner Process Extr Metall Rev, 2012, 33(6):391-403. doi: 10.1080/08827508.2011.601700 [4] LI C, WAN J H, SUN H H, LI L T. Investigation on the activation of coal gangue by a new compound method[J]. J Hazard Mater, 2010, 179(3):515-520. http://www.sciencedirect.com/science/article/pii/S0304389410003390 [5] QIAO X C, SI P, YU J G. A systematic investigation into the extraction of aluminum from coal spoil through kaolinite[J]. Environ Sci Technol, 2008, 42(22):8541-8546. doi: 10.1021/es801798u [6] ZHU P H, ZHENG M, ZHAO S Y, WU J Y, XU H X. A novel environmental route to ambient pressure dried thermal insulating silica aerogel via recycled coal gangue[J]. Adv Mater Sci Eng, 2016, 1-9. http://www.researchgate.net/publication/302634012_A_Novel_Environmental_Route_to_Ambient_Pressure_Dried_Thermal_Insulating_Silica_Aerogel_via_Recycled_Coal_Gangue [7] GUO W. Early hydration of composite cement with thermal activated coal gangue[J]. J Wuhan Univ Technol. 2010, 25(1):162-166. doi: 10.1007/s11595-010-1162-0 [8] ZHANG C S, LIU X F, WU Q S, DENG Y X, LI L. Study of mechanical force on coal gangue reactivity[J]. 2013, 539: 145-148. 10.4028/www.scientific.net/kem.539.145 [9] GENG J J, ZHOU M, LI Y X, CHEN Y C, HAN Y, WAN S, ZHOU X, HOU H B. Comparison of red mud and coal gangue blended geopolymers synthesized through thermal activation and mechanical grinding preactivation[J]. Constr Build Mater, 2017, 153:185-192. doi: 10.1016/j.conbuildmat.2017.07.045 [10] LI Y, YAO Y, LIU X M, SUN H H, NI W. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation[J]. Fuel, 2013, 109:527-533. doi: 10.1016/j.fuel.2013.03.010 [11] 肖汉敏, 马晓茜.污泥与煤和煤矸石共燃特性研究[J].燃料化学学报, 2008, 36(5): 545-550. http://www.cnki.com.cn/Article/CJFDTotal-RLHX200805006.htmXIAO Han-min, MA Xiao-qian. Technol characteristics of co-combustion of coal, coal gangue and sewage sludge[J]. 2008, 36(5): 545-550. http://www.cnki.com.cn/Article/CJFDTotal-RLHX200805006.htm [12] SALAHUDEEN N, AHMED A S, AL-MUHTASEB A H, DAUDA M, WAZIRI S M, JIBRIL B Y. Synthesis of gamma alumina from Kankara kaolin using a novel technique[J]. Appl Clay Sci, 2015, 105:170-177. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=85f3a973c3d5deda805356b55a48eee4 [13] 梅艳钢, 王志青, 方惠斌, 冯荣涛, 房倚天.燃烧与催化气化灰中铝溶出行为的研究[J].燃料化学学报, 2017, 45(4):394-399. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201704002MEI Yan-gang, WANG Zhi-qing, FANG Hui-bin, FENG Rong-tao, FANG Yi-tian. Comparison of leaching behaviors of aluminum in ash from combustion and catalytic gasification[J]. J Fuel Chem Technol, 2017, 45(4):394-399. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201704002 [14] MOLINO A, CHIANESE S, MUSMARRA D. Biomass gasification technology:The state of the art overview[J]. J Energy Chem, 2016, 25(1):10-25. http://www.cqvip.com/QK/84213A/201601/668981350.html [15] PARVEZ A M, AFZAL M T. Gasification performance of torrefied Timothy hay and spruce wood chars in a CO2 environment[J]. Cana J Chem Eng, 2020, 98(8):1696-1707. doi: 10.1002/cjce.23729 [16] 陈凡敏, 王兴军, 王西明, 周志杰.煤催化气化过程中钾的迁移及其对气化反应特性的影响[J].燃料化学学报, 2013, 41(3):265-270. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201303002CHEN Fan-min, WANG Xing-jun, WANG Xi-ming, ZHOU Zhi-jie. Transformation of potassium during catalytic gasification of coal and the effect on gasification[J]. J Fuel Chem Technol, 2013, 41(3):265-270. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201303002 [17] 孙雪莲, 王黎, 张占涛.煤气化复合催化剂研究及机理探讨[J].煤炭转化, 2006, 29(1):15-18. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=mtzh200601004SUN Xue-lian, WANG Li, ZHANG Zhan-tao. Study on compound catalyst for gasification and its mechanism[J].Coal Conv, 2006, 29(1):15-18. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=mtzh200601004 [18] 王勇, 樊红莉, 徐美玲, 李风海.煤催化气化催化剂及其催化机理研究进展[J].山东化工, 2015, 44(15):58-59. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sdhg201515023WANG Yong, FAN Hong-li, XU Mei-ling, LI Feng-hai. Research progress on catalysts and catalytic mechanism of coal catalytic gasification[J]. Shandong Chem Ind, 2015, 44(15):58-59. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sdhg201515023 [19] DONG L, LIANG X X, SONG Q, GAO G, SONG L H, SHU Y F, SHU X Q. Study on Al2O3 extraction from activated coal gangue under different calcination atmospheres[J]. J Therm Sci, 2017, 26(6):570-576. doi: 10.1007/s11630-017-0975-y [20] WANG Y W, WANG Z Q, HUANG J J, FANG Y T. Catalytic gasification activity of Na2CO3 and comparison with K2CO3 for a high-aluminum coal char[J]. Energy Fuels, 2015, 29(11):6988-6998. doi: 10.1021/acs.energyfuels.5b01537 [21] HUANG Y Q, YIN X L, WU C Z. Effects of metal catalysts on CO2 gasification reactivity of biomass char[J]. Biotechnol Adv, 2009, 27(5):568-572. doi: 10.1016/j.biotechadv.2009.04.013 [22] LI S F, CHENG Y L. Catalytic gasification of gas-coal char in CO2[J]. Fuel, 1995, 74(3):456-458. doi: 10.1016/0016-2361(95)93482-S [23] 张恒, 李俊国, 郭帅, 王志青, 张永奇, 房倚天.煤灰对玉米秸秆焦气化过程中固钾及其灰熔融性的研究[J].燃料化学学报, 2018, 46(9):1055-1062. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201809004ZHANG Heng, LI Jun-guo, GUO Shuai, WANG Zhi-qing, ZHANG Yong-qi, FANG Yi-tian. Influence of coal ash on potassium retention and ash fusibility during gasification of corn stalk coke[J]. J Fuel Chem Technol, 2018, 46(9):1055-1062. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201809004 [24] TURN S Q, KINOSHITA C M, ISHIMURA D M, ZHOU J, HIRAKI T T, MASUTANI S M. A review of sorbent materials for fixed bed alkali getter systems in biomass gasifier combined cycle power generation applications[J]. J Inst Energy, 1998, 71(489):163-177. http://www.researchgate.net/publication/283156645_A_review_of_sorbent_materials_for_fixed_bed_alkali_getter_systems_in_biomass_gasifier_combined_cycle_power_generation_applications [25] ZHOU C C, LIU G J, YAN Z C, FANG T, WANG R W. Transformation behavior of mineral composition and trace elements during coal gangue combustion[J]. Fuel, 2012, 97:644-650. doi: 10.1016/j.fuel.2012.02.027 [26] TANG J, WANG J. Catalytic steam gasification of coal char with alkali carbonates:A study on their synergic effects with calcium hydroxide[J]. Fuel Process Technol, 2016, 142:34-41. doi: 10.1016/j.fuproc.2015.09.020 [27] JIANG M Q, HU J, WANG J. Calcium-promoted catalytic activity of potassium carbonate for steam gasification of coal char:Effect of hydrothermal pretreatment[J]. Fuel, 2013, 109:14-20. doi: 10.1016/j.fuel.2012.06.100 [28] 管嵘清, 杜梅芳, 李洁, 陈玉爽, 张忠孝.煤灰中霞石与钠长石的光学性质对熔融特性影响[J].上海理工大学学报, 2010, 32(6):597-601. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=shlgdxxb201006021GUAN Rong-qing, DU Mei-fang, LI Jie, CHEN Yu-shuang, ZHANG Zong-xiao. Impact of optical properties of nepheline and albite onfusion characteristics in coal ash[J]. Univ Shanghai Sci Technol, 2010, 32(6):597-601. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=shlgdxxb201006021 [29] 李帆, 邱建荣, 郑楚光.煤中矿物质对灰熔融温度影响的三元相图分析[J].华中理工大学学报, 1996, 24(10):97-100. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199600254343LI Fan, QIU Jian-rong, ZHENG Chu-guang. The effect of mineral matter in coal on the ash melting point with ternary phase diagram[J]. J Huazhong Univ Sci Technol, 1996, 24(10):97-100. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199600254343 [30] WANG Y W, WANG Z Q, HUANG J J, FANG Y T. Improved catalyst recovery combined with extracting alumina from Na2CO3-catalyzed gasification ash of a high-aluminium coal char[J]. Fuel, 2018, 234:101-109. doi: 10.1016/j.fuel.2018.07.019 [31] FOO C T, MAHMOOD C S, SALLEH M A M. The study of aluminum loss and consequent phase transformation in heat-treated acid-leached kaolin[J]. Mater Charact, 2011, 62(4):373-377. doi: 10.1016/j.matchar.2011.01.017 [32] OKADA K, ARIMITSU N, KAMESHIMA Y, NAKAJIMA A, MACKENZIE K J D. Preparation of porous silica from chlorite by selective acid leaching[J]. Appl Clay Sci, 2005, 30(2):116-124. doi: 10.1016/j.clay.2005.04.001 [33] LIU M Z, YANG H M. Large surface area mesoporous Al2O3 from kaolin methodology and characterization[J]. Appl Clay Sci, 2010, 50(4):554-559. doi: 10.1016/j.clay.2010.10.012 -





 下载:
下载: