-
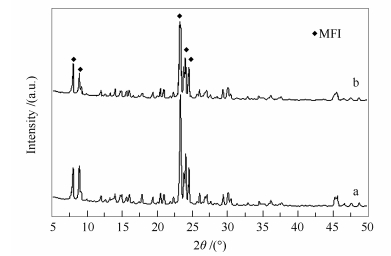
摘要: 采用二次生长法在多孔α-Al2O3载体上制备MFI型(ZSM-5和silicate-1)分子筛膜;通过XRD和SEM检测,证明所合成的分子筛膜为致密、交联和无取向的MFI型分子筛膜,厚度为5 μm;单组分气体渗透实验检测中,所制备样品膜的N2渗透量均小于10-11 mol/(m2·s·Pa),可认为其无缺陷;同时,考察了样品分子筛膜对H2S/CH4混合气的分离效果,在渗透压分别为0.3和0.5 MPa时,silicate-1分子筛膜的H2S/CH4的分离因子分别为1.99和4.44,而ZSM-5分子筛膜的CH4/H2S的分离因子分别为6.71和12.85。Abstract: The MFI (ZSM-5 and silicate-1) membranes with porous α-Al2O3 substrates were synthesized by secondary growth method. The results of scanning electron microscopy (SEM) and X-ray diffraction (XRD) indicate that the membranes with 5 μm thickness are composed of well-intergrown MFI crystals, which completely covers on the α-Al2O3 substrates in random orientation. The gas permeation measurements reveal that the resulting membranes are of high quality with few non-zeolitic pores. In addition, the separation properties of H2S/CH4 through the synthesized MFI membranes were investigated. Under the osmotic pressure of 0.3 and 0.5 MPa, the separation factors of H2S/CH4 by silicate-1 zeolite membrane are 1.99 and 4.44, and the separation factors of CH4/H2S by ZSM-5 zeolite membrane are 6.71 and 12.85, respectively.
-
Key words:
- MFI zeolite membrane /
- H2S/CH4 separation /
- secondary growth method
-
表 1 在不同压降下H2S/CH4混合气在silicalite-1和ZSM-5分子筛膜分离
Table 1 H2S/CH4 separating results for synthesized silicalite-1 zeolite membrane at different pressure drops
Zeolite membrane Osmotic pressure difference p/MPa Feed gas volume fraction/% Separation gas volume fraction/% Separation factor β H2S CH4 H2S CH4 Silicalite-1 0.3 30.51 69.49 18.24 82.76 1.99(H2S/CH4) 0.5 30.51 69.49 9.00 91.00 4.44(H2S/CH4) ZSM-5 0.3 44.37 55.63 84.25 15.75 6.71(CH4/H2S) 0.5 44.37 55.63 91.11 8.89 12.85(CH4/H2S) -
[1] LIN Y S, KUMAKIRI I, NAIR B N, ALSYOURI H. Microporous inorganic membranes[J]. Sep Purif Methods, 2002, 31(2):229-379. doi: 10.1081/SPM-120017009 [2] CARO J, NOACK M, KÖLSCH P, SCHÄFER R. Zealite membrane-state of their development and perspective[J]. Microporous Mesoporous Mater, 2000, 38(1):3-24. doi: 10.1016/S1387-1811(99)00295-4 [3] SNYDER M A, TSAPATSIS M. Hierarchical nanomanufacturing:from shaped zeolite nanoparticles to high performance separation membranes[J]. Angew Chem Int Ed, 2007, 46(40):7560-7573. doi: 10.1002/(ISSN)1521-3773 [4] XIA S X, PENG Y, LU H B, WANG Z B. The influence of nanoseeds on the pervaporation performance of MFI-type zeolite membranes on hollow fibers[J]. Microporous Mesoporous Mater, 2016, 222(1):128-137. http://www.sciencedirect.com/science/article/pii/S1387181115005557 [5] UENO K, NEGISHI H, OKUNO T, SAITO T, TAWARAYAMA H, ISHIKAWA S, MIYAMOTO M, UEMIYA S, SAWADA Y, OUMI Y. A simple secondary growth method for the preparation of silicalite-1 membrane on a tubular silica support via gel-free steam-assisted conversion[J]. J Membr Sci, 2017, 542(15):150-158. https://www.researchgate.net/publication/318925951_A_simple_secondary_growth_method_for_the_preparation_of_silicalite-1_membrane_on_a_tubular_silica_support_via_gel-free_steam-assisted_conversion [6] JIN S L, LEE Y J, TAE E L, YONG S P, YOON K B. Synthesis of zeolite as ordered multicrystal arrays[J]. Science, 2003, 301(5634):818-821. doi: 10.1126/science.1086441 [7] YAN Y, AND Z W. Controlling crystal orientation in zeolite MFI thin films by direct in situ crystallization[J]. Chem Mater, 2001, 13(3):1101-1107. doi: 10.1021/cm000849e [8] HEDLUNDA J, NOACKB M, KÖLSCH P, CREASERA D, CAROB J, STERTEA J. ZSM-5 membranes synthesized without organic templates using a seeding technique[J]. J Membr Sci, 1999, 159(1/2):263-273. http://www.sciencedirect.com/science/article/pii/S0376738899000691 [9] LAI R, GAVALAS G R. ZSM-5 membrane synthesis with organic-free mixtures[J]. Microporous Mesoporous Mater, 2000, 38(2/3):239-245. http://www.sciencedirect.com/science/article/pii/S1387181100001438 [10] UENO K, NEGISHI H, OKUNO T, SAITO T, TAWARAYAMA H, ISHIKAWA S, MIYAMOTO M, UEMIYA S, SAWADA Y, OUMI Y. High-performance silicalite-1 membranes on porous tubular silica supports for separation of ethanol/water mixtures[J]. Sep Purif Technol, 2017, 187(31):343-354. http://www.sciencedirect.com/science/article/pii/S1383586617309383 [11] LANG L, LIU X F, ZHANG B Q. Synthesis and characterization of h0h-oriented silicalite-1 films on α-Al2O3substrates[J]. Appl Surf Sci, 2008, 254(8):2353-2358. doi: 10.1016/j.apsusc.2007.09.031 [12] ARRUEB M, CORONAS J, MENÉNDEZ M, SANTAMARIA J. Separation of hydrocarbons from natural gas using silicalite membranes[J]. Sep Purif Technol, 2001, 25(1/3):275-286. http://www.sciencedirect.com/science/article/pii/S1383586601000545 [13] BEMAL M P, CORONAS J, MENÉNDEZ M, SANTAMARIA J. Separation of CO2/N2 mixtures using MFI-type zeolite membranes[J]. AIChE J, 2004, 50(1):127-135. doi: 10.1002/aic.10012 [14] MUREDDU M, FERINO I, ROMBI E, CUTRUFELLO M G, DEIANA P, ARDU A, MUSINU A, PICCALUGA G, CANNAS C. ZnO/SBA-15 composites for mid-temperature removal of H2S:Synthesis, performance and regeneration studies[J]. Fuel, 2012, 102:691-700. doi: 10.1016/j.fuel.2012.05.013 [15] HAFEZ M, MOHAMMAD S. Simultaneous separation of H2S and CO2 from CH4 by a high silica CHA-type zeolite membrane[J]. J Membr Sci, 2014, 470:159-165. doi: 10.1016/j.memsci.2014.07.025 [16] WEICHIH L, YUPEI C, CHINGPING T. Pilot-scale chemical-biological system for efficient H2S removal from biogas[J]. Bioresour Technol, 2013, 135:283-291. doi: 10.1016/j.biortech.2012.10.040 [17] ÁLVAREZ-Cruz R, SÁNCHEZ-FLORES B E, TORRES-GONZÁLEZ J, ANTAÑO-LÓPEZ R, CASTAÑEDA F. Insights in the development of a new method to treat H2S and CO2 from sour gas by alkali[J]. Fuel, 2012, 100:173-176. doi: 10.1016/j.fuel.2012.05.009 [18] ŽÁK M, BENDOVÁ H, FRIESS K, BARA J E, IZÁK P. Single-step purification of raw biogas to biomethane quality by hollow fiber membranes without any pretreatment-an innovation in biogas upgrading[J]. Sep Purif Technol, 2018, 203(12):36-40. http://www.sciencedirect.com/science/article/pii/S1383586618305574 [19] WANG S M, WU D, HUANG H L, YANG Q Y, TONG M M, LIU D H, ZHONG C L. Computational exploration of H2S/CH4 mixture separation using acid-functionalized UiO-66(Zr) membrane and composites[J]. Chin J Chem Eng, 2015, 23:1291-1299. doi: 10.1016/j.cjche.2015.04.017 [20] YANG W S, ZhANG B Q, LIU X F. Synthesis and characterization of SAPO-5 membranes on porous α-Al2O3 substrates[J]. Microporous Mesoporous Mater, 2009, 117:391-394. doi: 10.1016/j.micromeso.2008.07.015 [21] 杨文申, 郎林, 王凤旵, 阴秀丽, 吴创之. CoAPSO-5分子筛膜制备与CO2/CH4分离性能实验[J].农业机械学报, 2013, 44(9):114-117. https://www.doc88.com/p-9703556699510.htmlYANG Wen-shen, LANG Lin, WANG Feng-chan, YIN Xiu-li, WU Chuang-zhi. CO2/CH4 Separation Performance and CoAPSO-5 Zeolite Membrane Preparation[J]. Trans Chin Soc Agric Mach, 2009, 44(9):114-117. https://www.doc88.com/p-9703556699510.html -





 下载:
下载: