Differences in molecular composition of soluble organic species in two Chinese sub-bituminous coals with different reducibility
-
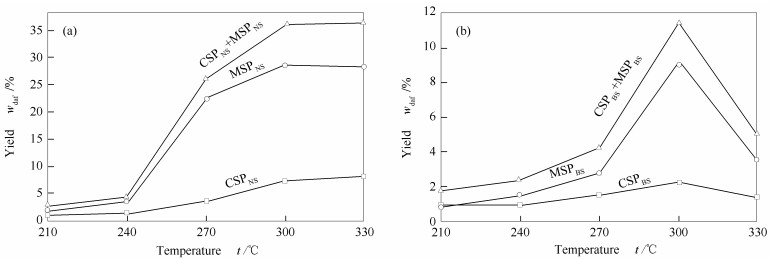
摘要: 本研究首先利用等体积二硫化碳/丙酮溶剂对弱还原性淖毛湖次烟煤(NS)和强还原性不连沟次烟煤(BS)进行萃取得到萃取物和萃余物(ERs),再利用环己烷和甲醇对ERs进行连续热溶得到热溶物(SPs)。NS和BS的萃取物产率分别为10.6%和8.0%,在300℃下NS和BS热溶物的总收率分别为36.3%和11.5%,这说明NS中存在更多可溶有机质。NS和BS萃取物中化合物均以芳烃为主。与BS相比,NS热溶物中脂肪烃和酚类的相对含量明显较高,萃取物中化合物的相对分子量分布范围较宽,而热溶物中化合物的相对分子量分布范围较窄。Abstract: Naomaohu sub-bituminous (NS) with weak reducibility and Buliangou sub-bituminous (BS) with strong reducibility were extracted in isometric carbon disulfide/acetone mixed solvent to get extracts and extraction residues (ERs). The ERs were thermal dissolved in cyclohexane and methanol to get soluble portions (SPs). The yields of the extracts from NS (ENS) and BS (EBS) are 10.6% and 8.0%, respectively, and the total yields of the SPs from NS and BS at 300℃ are 36.3% and 11.5%, respectively, indicating the solubility of the organic species in NS are better than that in BS. Arenes are the dominated compounds both in ENS and EBS. The relative contents of aliphatic hydrocarbons and phenols in the SPs of NS are obvious higher than those of BS. The molecular weight distribution of the compounds in ENS is wider than that in EBS, while the molecular weight distribution of the compounds in the SPs from NS is narrower than that from BS.
-
Key words:
- sub-bituminous coals /
- soluble organic species /
- molecular composition /
- thermal dissolution
-
Table 1 Proximate and ultimate analyses of coal samples
Sample Proximate analysis w/% ultimate analysis wdaf/% GR, I Rmaxo/% Qgr, maf/(MJ·kg-1) Mad Ad Vdaf C H N S O* NS 6.36 5.37 44.21 68.51 3.66 1.08 0.57 26.18 0 0.36 25.59 BS 2.01 17.66 35.95 77.50 4.62 1.27 0.84 15.77 0 0.79 23.32 Mad: moisture (air dried base); Ad: ash (dry base, i.e., moisture-free base); Vdaf: volatile matter (dry and ash-free base); *: by difference; GR, I: caking index; Rmaxo: average vitrinite reflectance; Qgr, maf: gross calorific value (moist ash-free base) Wavenumber σ/cm-1 Assignment 3415 phenolic-OH groups stretching vibration 2925 asymmetric stretching vibration of aliphatic C-H 2852 symmetric stretching vibration of aliphatic C-H 1612 stretching vibration of aromatic nucleus C=C 1454 asymmetric bending vibration of aliphatic C-H 1373 symmetric bending vibration of aliphatic C-H 1213 asymmetric stretching vibration of C-O 1082 symmetric stretching vibration of C-O 815, 752 out-of-plane deformation vibration of aromatic C-H Table 3 Arenes identified by GC/MS in CSPs and MSPs
Compound RC/% CSPNS, 300 CSPBS, 300 MSPNS, 300 MSPBS, 300 Toluene 2.27 21.06 Ethyl-benzene 1.70 12.71 2.94 4.77 m-xylene 5.44 27.46 4.44 2.35 Isopropyl-benzene 0.87 0.48 1.34 Propyl-benzene 0.43 Ethylmethylbenzene 1.90 0.26 3.01 2.69 1, 2, 3-trimethyl-benzene 0.46 2.53 7.77 1.71 Indan 1.12 Butyl-benzene 0.18 0.22 1-ethyl-2, 3-dimethyl-benzene 1.01 2.48 But-2-enyl-benzene 0.6 But-1-enyl-benzene 0.57 2.97 (2-methyl-propenyl)-benzene 0.78 1, 2, 4, 5-tetramethyl-benzene 0.09 0.15 (1-methyl-allyl)-benzene 0.12 2.97 1.38 3-methyl-1H-indene 0.28 Azulene 0.26 (1-methyl-but-1-enyl)-benzene 0.25 Naphthalene 0.48 1.31 Cyclopentyl-benzene 0.10 2, 3-dimethyl-1H-indene 1.49 Methylnaphthalene 0.88 1.84 2.96 1, 8-dimethyl-1, 2, 3, 4-tetrahydro-naphthalene 0.39 Heptyl-benzene 0.44 1, 2, 3-trimethyl-1H-indene 0.23 1-ethyl-naphthalene 0.09 2, 6-dimethyl-naphthalene 1.07 1.49 2.81 1.61 Diphenylmethane 0.14 Octyl-benzene 0.80 1, 1, 4, 5, 6-pentamethyl-indan 0.58 Nonyl-benzene 0.29 9H-fluorene 0.86 2-methyl-9H-fluorene 0.17 0.65 3-methyl-biphenyl 0.22 7-isopropyl-1-methyl-naphthalene 0.17 Decyl-benzene 0.15 4-isopropyl-1, 6-dimethyl-naphthalene 0.16 0.11 1, 4, 5, 8-tetramethyl-naphthalene 0.4 Phenanthrene 0.15 9-methylene-9H-fluorene 0.15 1-methyl-anthracene 0.40 2-methyl-phenanthrene 0.25 Total 23.93 52.36 29.39 41.18 -
[1] LI X, ASHIDA, R. MIURA K. Preparation of high-grade carbonaceous materials having similar chemical and physical properties from various low-rank coals by degradative solvent extraction[J]. Energy Fuels, 2012, 26(11):6897-6904. doi: 10.1021/ef301364p [2] SHUI H F, XU H Y, ZHOU Y, SHUI T, PAN C X, WANG Z C, LEI Z P, REN S B, KANG S G, XU C B. Study on hydro-liquefaction kinetics of thermal dissolution soluble fraction from Shenfu sub-bituminous coal[J]. Fuel, 2017, 200:576-582. doi: 10.1016/j.fuel.2017.03.048 [3] JONATHAN P M, CAROLINE B C, PAUL P. Interactions of Illinois No. 6 bituminous coal with solvents:A review of solvent swelling and extraction literature[J]. Energy Fuels, 2015, 29(3):1279-1294. doi: 10.1021/ef502548x [4] LI X, DEDY E P, RYUICHI A, KOUICHI M. Two-stage conversion of low-rank coal or biomass into liquid fuel under mild conditions[J]. Energy Fuels, 2015, 29:3127-3133. doi: 10.1021/ef502574b [5] SÖNMEZ Ö, GÖZMEN B, CEVIK T, GIRAY E S. Optimization of solvent extraction process of some turkish coals using response surface methodology and production of ash-free coal[J]. Asia-Pac J Chem Eng, 2016, 11(6):1001-1011. doi: 10.1002/apj.v11.6 [6] GRIFFITH J M, CLIFFORD C E B, RUDNICK L R, SCHOBERT H H. Solvent extraction of bituminous coals using light cycle oil:Characterization of diaromatic products in liquids[J]. Energy Fuels, 2009, 23(9):4553-4561. doi: 10.1021/ef9006092 [7] SHUI H F, WANG Z C, WANG G Q. Effect of hydrothermal treatment on the extraction of coal in the CS2/NMP mixed solvent[J]. Fuel, 2006, 85:1798-1802. doi: 10.1016/j.fuel.2006.02.005 [8] SÖNMEZ Ö, GIRAYE S. Producing ashless coal extracts by microwave irradiation[J]. Fuel, 2011, 90(6):2125-2131. doi: 10.1016/j.fuel.2011.02.006 [9] JANUSZ P, LUKASZ S. Effects of pressure on hydrogen transfer from tetralin to coal macerals[J]. Energy Fuels, 2005, 19:348-352. doi: 10.1021/ef040053s [10] ZHAO Y P, XIAO J, DING M, EDDINGS E G, WEI XY, FAN X, ZONG Z M. Sequential extraction and thermal dissolution of Baiyinhua lignite in isometric CS2/acetone and toluene/methanol binary solvents[J]. Energy Fuels, 2016, 30(1):47-53. doi: 10.1021/acs.energyfuels.5b01775 [11] DING M, ZHAO Y P, DOU Y Q, WEI X Y, FAN X, CAO J P, WANG Y L, ZONG Z M. Sequential extraction and thermal dissolution of Shengli lignite[J]. Fuel Process Technol, 2015, 135:20-24. doi: 10.1016/j.fuproc.2014.09.031 [12] LU H Y, WEI X Y, YU R, PENG Y L, QI X Z, QIE L M, WEI Q, LV J, ZONG Z M, ZHAO W, ZHAO Y P, NI Z H, WU L. Sequential thermal dissolution of Huolinguole lignite in methanol and ethanol[J]. Energy Fuels, 2011, 25(6):2741-2745. doi: 10.1021/ef101734f [13] ZHAO Y P, HU H Q, JIN L J, WU B, ZHU S W. Pyrolysis behavior of weakly reductive coals from northwest China[J]. Energy Fuels, 2009, 23:870-875. doi: 10.1021/ef800831y [14] WU B HU H Q, HUANG S P, FANG Y M, LI X, MENG M. Extraction of weakly reductive and reductive coals with sub-and supercritical water[J]. Energy Fuels, 2008, 22:3944-3948. doi: 10.1021/ef8002872 [15] CHANG H Z, WANG C G, ZENG F G, LI J, LI W Y, XIE K C. XPS comparative analysis of coal macerals with different reducibility[J]. J Fuel Chem Technol, 2006, 34(4):389-394. http://cn.bing.com/academic/profile?id=7459d7a0138a9e0bb1b4b0e0309945f6&encoded=0&v=paper_preview&mkt=zh-cn [16] ZOU X W, QIN T F, HUANG L H, ZHANG X L, YANG Z, WANG Y. Mechanisms and main regularities of biomass liquefaction with alcoholic solvents[J]. Energy Fuels, 2009, 23(10):5213-5218. doi: 10.1021/ef900590b [17] TIAN B, QIAO Y Y, TIAN Y Y, XIE K C, LIU Q, ZHOU H F. FT-IR study on structural changes of different-rank coals caused by single/multiple extraction with cyclohexanone and NMP/CS2 mixed solvent[J]. Fuel Process Technol, 2016, 154:210-218. doi: 10.1016/j.fuproc.2016.08.035 [18] CANEL M MISIRLIOGLU Z, CANEL E, BOZKURT P A. Distribution and comparing of volatile products during slow pyrolysis and hydropyrolysis of Turkish lignites[J]. Fuel, 2016, 186:504-517. doi: 10.1016/j.fuel.2016.08.079 [19] XIE X, ZHAO Y, QIU P H, LIN D, QIAN J, HOU H M, PEI J T. Investigation of the relationship between infrared structured and pyrolysis reactivity of coals with different ranks[J]. Fuel, 2018, 216:521-530. doi: 10.1016/j.fuel.2017.12.049 [20] SONG H J, LIU G R, ZHANG J Z, WU J H. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method[J]. Fuel Process Technol, 2017, 156:454-460. doi: 10.1016/j.fuproc.2016.10.008 [21] MICHAEL S, THOMAS A. Pyrolysis studies on the structure of ethers and phenols in coal[J]. Fuel, 1983, 62:1321-1326. doi: 10.1016/S0016-2361(83)80017-9 [22] VACLAVIK L, CAJKA T, HRBEK V, HAJSLOVA J. Ambient mass spectrometry employing direct analysis in real time (DART) ion source for olive oil quality and authenticity assessment[J]. Anal Chim Acta, 2009, 645(1/2):56-63. http://cn.bing.com/academic/profile?id=f113cd3925fc16849e5b49d712cb4d53&encoded=0&v=paper_preview&mkt=zh-cn [23] WANG Y, LI C M, HUANG L, LIU L, GUO Y L, MA L, LIU S Y. Rapid identification of traditional Chinese herbal medicine by direct analysis in real time (DART) mass spectrometry[J]. Anal Chim Acta, 2014, 845:70-76. doi: 10.1016/j.aca.2014.06.014 [24] CHERNETSOVA E S, BOCHKOV P O, OVCHAROV M V, ZHOKHOV S S, ABRAMOVICH R A. DART mass spectrometry:A fast screening of solid pharmaceuticals for the presence of an active ingredient, as an alternative for IR spectroscopy[J]. Drug Test Anal, 2010, 2(5/6):292-294. http://cn.bing.com/academic/profile?id=1fbb02b5d1b7c3b6ef7fb3d736765186&encoded=0&v=paper_preview&mkt=zh-cn [25] CRAWFORD E, MUSSELMAN B. Evaluating a direct swabbing method for screening pesticides on fruit and vegetable surfaces using direct analysis in real time (DART) coupled to an exactive benchtop orbitrap mass spectrometer[J]. Anal Bioanal Chem, 2012, 403(10):2807-2812. doi: 10.1007/s00216-012-5853-6 [26] FAN X, WANG C F, YOU C Y, WEI X Y, CHEN L, CAO J P, ZHAO Y P, ZHAO W, WANG Y G, LU J L. Characterization of a Chinese lignite and the corresponding derivatives using direct analysis in real time quadrupole time-of-flight mass spectrometry[J]. RSC Adv, 2016, 6:105780-105785. doi: 10.1039/C6RA23899H [27] WANG C F, FAN X, ZHANG F, WANG S Z, ZHAO Y P, ZHAO X Y, ZHAO W, ZHU T G, LU J L, WEI X Y. Characterization of humic acids extracted from a lignite and interpretation for the mass spectra[J]. RSC Adv, 2017, 7:20677-20684. doi: 10.1039/C7RA01497J -





 下载:
下载: