Fabrication of effective desulfurization species active sites in the CeY zeolites and the adsorption desulfurization mechanisms
-
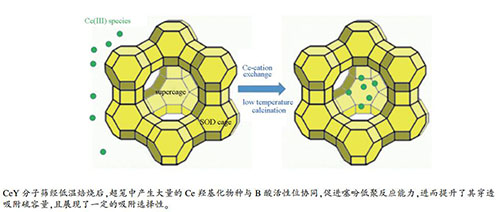
摘要: 以不同焙烧温度和Ce负载量的CeY分子筛为研究对象,运用XRD及N2吸附表征其织构性质;运用吡啶吸附红外光谱法剖析了分子筛中活性位的化学属性;采用固定床评价其对噻吩模拟油的吸附脱硫性能及芳烃和烯烃对噻吩脱除的影响;并结合红外光谱和GC-SCD技术分析了其脱硫机制。结果表明,CeY样品经150 ℃焙烧后,其超笼中具备高含量的B酸和Ce羟基化物种活性位,两者协同增强了噻吩低聚反应能力,进而提高了其吸附穿透硫容量(18.45 mg(S)/g);而提升焙烧温度和Ce负载量会严重降低其有效活性位的数量,削弱了噻吩低聚反应能力,其吸附穿透硫容量显著减小(4.03 mg(S)/g)。当加入烯烃和芳烃后,CeY-12.3-150吸附剂对含低浓度(质量分数)1-己烯(< 1.0%)和苯(< 0.1%)的噻吩模拟油依旧保持较高吸附穿透硫容量;但随两者含量的持续增加,其硫容量急剧下降。其主要分别归因于噻吩烷基化反应的发生及"S-H"键的作用模式。Abstract: A series of CeY zeolites with different cerium loadings and calcined at different temperatures were prepared and used as the adsorbent for the desulfurization of thiophene containing model oil. The CeY zeolites were characterized by XRD, N2 sorption, FT-IR spectroscopy and GC-SCD and GC-MSD techniques. The effects of aromatics and olefins on the adsorption desulfurization performance were investigated and the active species and reaction mechanism for the adsorption desulfurization on CeY zeolites were probed. The results indicate that the CeY zeolite calcined at 150 ℃ is provided with a large number of Brönsted acid sites and hydroxylated cerium species in the supercages, which can synergistically promote the thiophene oligomerization and then enhance the sulfur breakthrough adsorption capacity (18.45 mg (S)/g). However, a further increase in the calcination temperature and cerium loading may greatly reduce the number of active sites for the adsorption desulfurization and suppress the thiophene oligomerization reaction, leading to a significant decrease in the sulfur breakthrough adsorption capacity (4.03 mg (S)/g). For the thiophene model oils containing low concentration of 1-hexene (< 1.0%) or benzene (< 0.1%), the CeY-12.3-150 zeolite (with a cerium loading of 12.3% and calcined at 150 ℃) also exhibits a relatively high sulfur breakthrough adsorption capacity. However, a further increase in the content of 1-hexene or benzene in the feed may lead to a sharp decrease in the sulfur breakthrough adsorption capacity, due to the alkylation of thiophene and the adsorption mode of "S-H" bonding.
-
图 4 噻吩模拟油在CeY分子筛上的吸附穿透曲线(a)及穿透吸附硫容量(b)
Figure 4 Breakthrough curves of thiophene adsorption in a fixed-bed reactor on various CeY zeolites with a liquid feed containing 300 mg (S)/kg sulfur in n-octane at room temperature (a) and a comparison of various CeY zeolites in their sulfur breakthrough adsorption capacity (b)
图 8 含不同苯质量分数的噻吩模拟油在CeY-12.3-150分子筛上的噻吩吸附穿透曲线(a), 苯吸附穿透曲线(b)及穿透吸附硫容量(c)
Figure 8 Breakthrough curves of thiophene (a) and benzene (b) adsorption in a fixed-bed reactor on the CeY-12.3-150 zeolite adsorbent with a liquid feed containing 300 mg (S)/kg sulfur thiophene and different benzene concentrations in n-octane at room temperature and the corresponding sulfur breakthrough adsorption capacities (c)
图 9 含不同1-己烯质量分数的噻吩模拟油在CeY-12.3-150分子筛上的噻吩吸附穿透曲线(a), 1-己烯吸附穿透曲线(b)及其穿透吸附硫容量(c)
Figure 9 Breakthrough curves of thiophene (a) and 1-hexene (b) adsorption in a fixed-bed reactor on the CeY-12.3-150 zeolite adsorbent with a liquid feed containing 300 mg (S)/kg sulfur thiophene and different 1-hexene concentrations in n-octane at room temperature and the corresponding sulfur breakthrough adsorption capacities (c)
图 10 含0.01%(a)和20.0%(b)1-己烯的噻吩模拟油在CeY-12.3-150分子筛上吸附脱硫过程中不同吸附时间段液相产物的GC-SCD谱图
Figure 10 GC-SCD chromatograms of the sulfur compounds in liquid products during the adsorption desulfurization process in a fixed-bed reactor on the CeY-12.3-150 zeolite with a liquid feed containing 300 mg (S)/kg sulfur thiophene and 0.01% (a) and 20.0% (b) 1-hexene in n-octane
表 1 NaY和CeY分子筛的结构参数
Table 1 Textural properties of the NaY and CeY zeolites
Sample Ce loading w/% I12.5/I11.9 Surface area A /(m2·g-1) Pore volume v /(cm3·g-1) ABET Amicro Ameso vtotal vmicro vmeso NaY - 0 585.6 551.2 34.4 0.34 0.29 0.05 CeY-12.3-150 12.3 3.86 436.0 389.9 46.1 0.24 0.20 0.04 CeY-12.3-550 12.3 0.65 575.7 518.1 57.6 0.33 0.26 0.07 CeY-17.4-550 17.4 0.83 556.5 476.1 80.4 0.31 0.24 0.07 -
[1] 杨永坛, 杨海鹰, 宗保宁, 陆婉珍.催化裂化汽油中硫化物的气相色谱-原子发射光谱分析方法及应用[J].分析化学, 2003, 31(10):1153-1158. doi: 10.3321/j.issn:0253-3820.2003.10.001YANG Yong-tan, YANG Hai-ying, ZONG Bao-ning, LU Wan-zhen. Determination and distribution of sulfur compounds in gasoline by gas chromatography-atomic emission detector[J]. Chin J Anal Chem, 2003, 31(10):1153-1158. doi: 10.3321/j.issn:0253-3820.2003.10.001 [2] 杨永坛, 王征.焦化汽油中硫化物类型分布的气相色谱-硫化学发光检测方法[J].色谱, 2007, 25(3):384-388. doi: 10.3321/j.issn:1000-8713.2007.03.021YANG Yong-tan, WANG Zheng. Determination and distribution of sulfur compounds in coked gasoline by gas chromatography-sulfur chemiluminescence detection[J]. Chin J Chromatogr, 2007, 25(3):384-388. doi: 10.3321/j.issn:1000-8713.2007.03.021 [3] 朱丽君, 夏道宏, 项玉芝, 周玉路.加氢焦化汽油硫化合物组成分析[J].石油与天然气化工, 2009, 38(6):494-497. doi: 10.3969/j.issn.1007-3426.2009.06.009ZHU LI-jun, XIA Dao-hong, XIANG Yu-zhi, ZHOU Yu-lu. Composition analysis of sulfur compounds in hydrocoking gasoline[J]. Chem Eng Oil Gas, 2009, 38(6):494-497. doi: 10.3969/j.issn.1007-3426.2009.06.009 [4] SONG C S. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J]. Catal Today, 2003, 86(1):211-263. doi: 10.1016-S0920-5861(03)00412-7/ [5] DEHGHAN R, ANBIA M. Zeolites for adsorptive desulfurization from fuels:A review[J]. Fuel Process Technol, 2017, 167:99-116. doi: 10.1016/j.fuproc.2017.06.015 [6] TAN P, JIANG Y, SUN L B, LIU X Q, ALBAHILY K, RAVON U, VINU A. Design and fabrication of nanoporous adsorbents for the removal of aromatic sulfur compounds[J]. J Mater Chem A, 2018, 6(47):23978-24012. doi: 10.1039/C8TA09184F [7] VELU S, MA X L, SONG C S. Selective adsorption for removing sulfur from jet fuel over zeolite-based adsorbents[J]. Ind Eng Chem Res, 2003, 42(21):5293-5304. doi: 10.1021/ie020995p [8] LIN L G, ZHANG Y Z, ZHANG H Y, LU F W. Adsorption and solvent desorption behavior of ion-exchanged modified Y zeolites for sulfur removal and for fuel cell applications[J]. J Colloid Interf Sci, 2011, 360(2):753-759. doi: 10.1016/j.jcis.2011.04.075 [9] WANG H G, SONG L J, JIANG H, XU J, JIN L L, ZHANG X T, SUN Z L. Effects of olefin on adsorptive desulfurization of gasoline over Ce(Ⅳ)Y zeolites[J]. Fuel Process Technol, 2009, 90(6):835-838. doi: 10.1016/j.fuproc.2009.03.004 [10] DUAN L H, GAO X H, MENG X H, ZHANG H T, WANG Q, QIN Y C, ZHANG X T, SONG L J. Adsorption, co-adsorption, and reactions of sulfur compounds, aromatics, olefins over Ce-exchanged Y zeolite[J]. J Phys Chem C, 2012, 116(49):25748-25756. doi: 10.1021/jp303040m [11] QIN Y C, MO Z S, YU W G, DONG S W, DUAN L H, GAO X H, SONG L J. Adsorption behaviors of thiophene, benzene, and cyclohexene on FAU zeolites:Comparison of CeY obtained by liquid-, and solid-state ion exchange[J]. Appl Surf Sci, 2014, 292:5-15. doi: 10.1016/j.apsusc.2013.11.036 [12] ZU Y, ZHANG C, QIN Y C, ZHANG X T, ZHANG L, LIU H H, GAO X H, SONG L J. Ultra-deep adsorptive removal of thiophenic sulfur compounds from FCC gasoline over the specific active sites of CeHY zeolite[J]. J Energy Chem, 2019, 39:256-267. doi: 10.1016/j.jechem.2019.04.010 [13] ZU Y, HUI Y, QIN Y C, ZHANG L, LIU H H, ZHANG X T, GUO Z S, SONG L J, GAO X H. Facile Fabrication of effective cerium (Ⅲ) hydroxylated species as adsorption active sites in CeY zeolite adsorbents towards ultra-deep desulfurization[J]. Chem Eng J, 2019, 375:122014. doi: 10.1016/j.cej.2019.122014 [14] SHI Y C, ZHANG W., ZHANG H X, TIAN F P, JIA C Y, CHEN Y Y. Effect of cyclohexene on thiophene adsorption over NaY and LaNaY zeolites[J]. Fuel Process Technol, 2013, 110:24-32. doi: 10.1016/j.fuproc.2013.01.008 [15] SHI Y C, YANF X J, TIAN F P, JIA C Y, CHEN Y Y. Effects of toluene on thiophene adsorption over NaY and Ce(Ⅳ)Y zeolites[J]. J Nat Gas Chem, 2012, 21(4):421-425. doi: 10.1016/S1003-9953(11)60385-X [16] 王祥生, 罗国华. HZSM-5沸石上焦化苯的精制脱硫[J].催化学报, 1996, 17(6):530-534.WANG Xiang-sheng, LUO Guo-hua. The removal of thiophene from coking benzene over HZSM-5 zeolite[J]. Chin J Catal, 1996, 17(6):530-534. [17] WANG J, XU F, XIE W J, MEI J Z, ZHANG Q Z, CAI J, CAI W M. The enhanced adsorption of dibenzothiophene onto cerium/nickel-exchanged zeolite Y[J]. J Hazard Mater, 2009, 163(2/3):538-543. [18] 秦玉才, 高雄厚, 段林海, 范跃超, 于文广, 张海涛, 宋丽娟.酸催化及竞争吸附对CeY分子筛吸附脱硫性能的影响[J].物理化学学报, 2014, 30(3):544-550. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb201403021QIN Yu-cai, GAO Xiong-hou, DUAN Lin-hai, FAN Yue-chao, YU Wen-guang, ZHANG Hai-tao, SONG Li-juan. Effects on adsorption desulfurization of CeY zeolites:Acid catalysis and competitive adsorption[J]. Acta Phys-Chim Sin, 2014, 30(3):544-550. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb201403021 [19] LI J C, ZENG P H, ZHAO L, REN S Y, GUO Q X, ZHANG H J, WANG B J, LIU H H, PANG X M, GAO X H, SHEN B J. Tuning of acidity in CeY catalytic cracking catalysts by controlling the migration of Ce in the ion exchange step through valence changes[J]. J Catal, 2015, 329:441-448. doi: 10.1016/j.jcat.2015.06.012 [20] LIAO J J, BAO W R, CHANG L P. An approach to study the desulfurization mechanism and the competitive behavior from aromatics:A case study on CeY zeolite[J]. Fuel Process Technol, 2015, 140:104-112. doi: 10.1016/j.fuproc.2015.08.036 [21] LIAO J J, WANG Y, CHANG L P, BAO W R. A process for desulfurization of coking benzene by a two-step method with reuse of sorbent/thiophene and its key procedures[J]. Green Chem, 2015, 17(5):3164-3175. doi: 10.1039/C4GC02505A [22] DU X H, GAO X H, Zhang H T, LI X L, LIU P S. Effect of cation location on the hydrothermal stability of rare earth-exchanged Y zeolites[J]. Catal Commun, 2013, 35:17-22. doi: 10.1016/j.catcom.2013.02.010 [23] KIM C W, KANG H C, HEO N H, SEFF K. Encapsulating photoluminescent materials in zeolites. Ⅱ. Crystal structure of fully dehydrated Ce21H46O18-Y (Si/Al=1.69) containing Ce4O44+, CeOH2+, Ce3+, and H+[J]. J Phys Chem C, 2015, 119(43):24501-24511. doi: 10.1021/acs.jpcc.5b08373 [24] LEE E F T, REES L V C. Calcination of cerium (Ⅲ) exchanged Y zeolite[J]. Zeolites, 1987, 7(5):446-450. doi: 10.1016/0144-2449(87)90013-3 [25] NERJ J G, MASCARENHAS Y P, BONAGAMBA T J, Mello N C, SOUZA-AGUIAR E F. Location of cerium and lanthanum cations in CeNaY and LaNaY after calcination[J]. Zeolites, 1997, 18:44-49. doi: 10.1016/S0144-2449(96)00094-2 [26] BOLTON A P. The nature of rare-earth exchanged Y zeolites[J]. J Catal, 1971, 22(1):9-15. doi: 10.1016/0021-9517(71)90259-4 [27] WANG N N, WANG Y, CHENG H F, FU M E, TAO Z, WU W Z. Relationship between two characteristic diffractions and the status of cationic lanthanum species in zeolite LaNaY[J]. J Porous Mater, 2013, 20(5):1371-1378. doi: 10.1007/s10934-013-9723-1 [28] QIU L M, FU Y, ZHENG J Y, HUANG N G, LU L J, GAO X Z, XIN M D, LUO Y B, SHI Y Q, XU G T. Investigation on the cation location, structure and performances of rare earth-exchanged Y zeolite[J]. J Rare Earths, 2017, 35(7):658-666. doi: 10.1016/S1002-0721(17)60960-8 [29] WANG M, JAEGERS N R, LEE M S, WAN C, HU J Z, SHI H, MEI D H, BURTON S D, CAMAIONI D M, GUTIERREZ O Y, GLEZAKOU V A, ROUSSEAU R, WANG Y, LERCHER J A. Genesis and stability of hydronium ions in zeolite channels[J]. J Am Chem Soc, 2019, 141(8):3444-3455. doi: 10.1021/jacs.8b07969 [30] 祖运, 秦玉才, 高雄厚, 莫周胜, 张磊, 张晓彤, 宋丽娟.催化裂化条件下噻吩与改性Y分子筛的作用机制[J].燃料化学学报, 2015, 43(7):862-869. doi: 10.3969/j.issn.0253-2409.2015.07.012ZU Yun, QIN Yu-cai, GAO Xiong-hou, MO Zhou-sheng, ZHANG Lei, ZHANG Xiao-tong, SONG Li-juan. Mechanisms of thiophene conversion over the modified Y zeolites under catalytic cracking conditions[J]. J Fuel Chem Technol, 2015, 43(7):862-869. doi: 10.3969/j.issn.0253-2409.2015.07.012 -





 下载:
下载: