Synthesis of core-shell structured MFI-type composite zeolites by isomorphous epitaxial growth
-
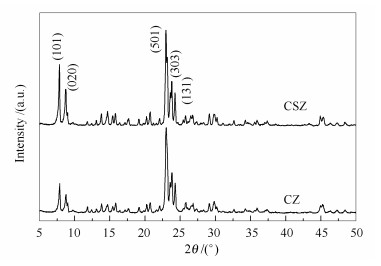
摘要: 采用二次热液结晶法,以四丙基氢氧化铵水溶液预处理过的低硅ZSM-5分子筛为晶核,通过调控pH值、水量和晶化时间等二次结晶条件,在晶核上外延生长了高硅ZSM-5壳,制备了MFI/MFI核壳型复合分子筛。通过X射线衍射、扫描电镜、能量色散谱仪、透射电子显微镜、N2吸附-脱附和NH3-程序升温脱附等手段表征了所合成的核壳分子筛的晶体结构、表面形态及核/壳界面,并对它们的结构参数以及酸性进行了初步评估。结果表明,核壳复合分子筛的壳层由多层200 nm的MFI沸石晶粒组成;高硅ZSM-5分子筛壳层的生成,引入了介孔结构,显著增大了外比表面积;同时,核壳结构的形成降低了复合分子筛酸性和外表面的酸密度,但增加了弱酸量。当二次晶化母液pH值为8.5,H2O/SO2物质的量比为30,晶化时间为24 h时,高硅分子筛壳层更易可控生长。Abstract: MFI/MFI core-shell composite zeolites with a low-silica ZSM-5 core and a high-silica shell were successfully synthesized by secondary hydrothermal crystallization on the low-silica ZSM-5 cores that was pretreated with a basic TPAOH aqueous solution; the preparation parameters for shell growth including the pH value, water amount, and crystallization time were well considered. The crystal structure, surface morphology, core/shell interface, textural properties and surface acidity of the resultant core-shell zeolites were characterized by X-ray diffraction, scanning electron microscopy, energy dispersive spectrometer, transmission electron microscopy, N2 physisorption and NH3 temperature programmed desorption. The results indicated that in the core-shell composite zeolites, high-silica ZSM-5 shell with a particle size of 200 nm is well developed on the surface of core crystal. The growth of ZSM-5 shell results in an increase of external surface area, decrease of external acid density and increase of weak acid sites without influencing pore structure. The isomorphous epitaxial growth of high-silica ZSM-5 shells can be effectively controlled under the conditions of a pH value of 8.5, H2O/SiO2 mol ratio of 30 and crystallization for 24 h during the secondary crystallization.
-
Key words:
- ZSM-5 /
- secondary crystallization /
- core/shell structure /
- composite zeolite /
- surface acidity
-
表 1 分子筛的织构性质
Table 1 Textural properties of the CZ and CSZ samples
Sample ABET /(m2·g-1) Amicroa /(m2·g-1) Aextermalb /(m2·g-1) vtotalc /(cm3·g-1) vmicrod /(cm3·g-1) CZ 249 208 41.0 0.173 0.095 9 CSZ 353 198 155.0 0.192 0.089 7 a, b, d: calculated by t-plot method; c: calculated at p/p0=0.995 表 2 不同pH值条件下合成分子筛的晶胞参数
Table 2 Unit cell parameters of the zeolites prepared under different pH values
pH value Unit cell parameters /nm Volume
V/nm3a b c 5.1 2.016 2.017 1.349 5.486 6.7 2.019 2.026 1.358 5.556 8.5 2.014 2.013 1.349 5.471 12.5 2.020 2.017 1.353 5.511 表 3 不同水量条件下合成分子筛的晶胞参数
Table 3 Unit cell parameters of the zeolites prepared with different contents of H2O
H2O/SO2
(mol ratio)Unit cell parameters /nm Volume
V/nm3a b c 30 2.015 2.017 1.359 5.525 80 2.020 2.026 1.353 5.536 150 2.015 2.022 1.359 5.537 250 2.019 2.026 1.358 5.556 表 4 不同二次晶化时间下合成分子筛的晶胞参数
Table 4 Unit cell parameters the zeolites obtained with different crystallization times
Time t/h Unit cell parameters /nm Volume
V/nm3a b c 12 2.016 2.017 1.354 5.550 24 2.012 2.013 1.350 5.468 36 2.017 2.031 1.359 5.566 48 2.015 2.017 1.359 5.525 -
[1] OKAMOTO M, OSAFUNE Y. MFI-type zeolite with a core-shell structure with minimal defects synthesized by crystal overgrowth of aluminum-free MFI-type zeolite on aluminum-containing zeolite and its catalytic performance[J]. Microporous Mesoporous Mater, 2011, 143(2/3): 413-418. https://www.researchgate.net/publication/251673974_MFI-type_zeolite_with_a_core-shell_structure_with_minimal_defects_synthesized_by_crystal_overgrowth_of_aluminum-free_MFI-type_zeolite_on_aluminum-containing_zeolite_and_its_catalytic_performance [2] 张佩珊, 马波, 杨卫亚, 凌凤香, 沈智奇, 王少军, 候宇鑫.核壳结构Beta/MCM-22双微孔复合分子筛的合成与表征[J].燃料化学学报, 2014, 42(10): 1240-1245. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18509.shtmlZHANG Pei-shan, MA Bo, YANG Wei-ya, LING Feng-xiang, SHEN Zhi-qi, WANG Shao-jun, HOU Yu-xin. Synthesis and characterization of core-shell Beta/MCM-22 micro-microporous composite zeolites[J]. J Fuel Chem Technol, 2014, 42(10): 1240-1245. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18509.shtml [3] LI K H, VALLA J, GARCIA-MARTINEZ J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking[J]. ChemCatChem, 2014, 6(1): 46-66. doi: 10.1002/cctc.v6.1 [4] GORA L, SULIKOWSKI B, SERWICKA E M. Formation of structured silicalite-1/ZSM-5 composites by a self-assembly process[J]. Appl Catal A: Gen, 2007, 325(2): 316-321. doi: 10.1016/j.apcata.2007.02.047 [5] BOUIZI Y, ROULEAU L, VALTCHEV V P. Factors controlling the formation of core-shell zeolite-zeolite composites[J]. Chem Mater, 2006, 18: 4959-4966. doi: 10.1021/cm0611744 [6] VU D V, MIYAMOTO M, NISHIYAMA N, ICHIKAWAB S, EGASHIRAA Y, UEYAMA K. Catalytic activities and structures of silicalite-1/H-ZSM-5 zeolite composites[J]. Microporous Mesoporous Mater, 2008, 115(1/2): 106-112. https://www.researchgate.net/profile/Korekazu_Ueyama/publication/248293134_Catalytic_activities_and_structures_of_silicalite-1H-ZSM-5_zeolite_composites/links/0f31753bd41068ed65000000.pdf [7] ZHAN Y Z, LI X X, ZHANG Y G, HAN L, CHEN Y L. Phase and morphology control of LTA/FAU zeolites by adding trace amounts ofinorganicions[J]. Ceram Int, 2013, 39(5): 5997-6003. doi: 10.1016/j.ceramint.2013.01.005 [8] KONG D J, ZHENG J L, YUAN X H, WANG Y D, FANG D Y. Fabrication of core/shell structure via overgrowth of ZSM-5 layers on mordenite crystals[J]. Microporous Mesoporous Mater, 2009, 119(1/2/3): 91-96. https://www.researchgate.net/publication/248293302_Fabrication_of_coreshell_structure_via_overgrowth_of_ZSM-5_layers_on_mordenite_crystals [9] ROLLMANN L D. ZSM-5 containing aluminum-free shells on its surface: US, 4088605[P]. 1978-05-09. [10] 孔德金, 邹薇, 童伟益, 房鼎业. MFI/MFI核壳分子筛的合成及催化性能研究[J].化学学报, 2009, 67(15): 1765-1770.KONG De-jin, ZOU Wei, TONG Wei-yi, FANG Ding-ye. Synthesis and catalytic behaviors of MFI/MFI core-shell zeolite[J]. Acta Chim Sin, 2009, 67(15): 1765-1770. [11] LIN Y S, DUKE M C. Recent progress in polycrystalline zeolite membrane research[J]. Curr Opin Chem Eng, 2013, 2(2): 209-216. doi: 10.1016/j.coche.2013.03.002 [12] LI Q H, WANG Z, HEDLND J, CREASER D, ZHANG H, ZOU Y D, BONS A J. Synthesis and characterization of colloidal zoned MFI crystals[J]. Microporous Mesporous Mater, 2005, 78(1): 1-10. doi: 10.1016/j.micromeso.2004.09.010 [13] GUO Y P, WANG H J, GUO Y J, GUO L H, CHU L F, GUO C X. Fabrication and characterization of hierarchical ZSM-5 zeolites by using organosilanes as additives[J]. Chem Eng J, 2011, 166(1): 391-400. doi: 10.1016/j.cej.2010.10.057 [14] GROEN J C, PEFFER L A A, MOULIJIN J A, PÉREZ-RAMÍREZ J. Mesoporosity development in ZSM-5 zeolite upon optimized desilication conditions in alkaline medium[J]. Colloids Surf A, 2013, 241(1/2/3): 53-58. https://www.researchgate.net/publication/229098413_Mesoporosity_development_in_ZSM-5_zeolite_upon_optimized_desilication_conditions_in_alkaline_medium [15] VU D V, MIYAMOTO M, NISHIYAMA N, EGASHIRA Y, UEYAMA K. Selective formation of para-xylene over H-ZSM-5 coated with polycrystalline silicalite crystals[J]. J Catal, 2006, 243(2): 389-394. doi: 10.1016/j.jcat.2006.07.028 [16] GROEN J C, JANSEN J C, MOULIJIN J A, PÉREZ-RAMÍREZ J. Optimal aluminum-assisted mesoporosity development in MFI zeolites by desilication[J]. J Phys Chem B, 2004, 108: 13062-13065. doi: 10.1021/jp047194f [17] DONK S V, JANSSEN A H, BITTER J H, JONG K P. Generation, characterization, and impact of mesopores in zeolite catalysts[J]. Catal Rev, 2003, 45: 297-319. doi: 10.1081/CR-120023908 [18] CHANG C D, BELL A T. Studies on the mechanism of ZSM-5 formation[J]. Catal Lett, 1991, 8(5): 305-316. https://www.researchgate.net/publication/227106848_Studies_on_the_mechanism_of_ZSM-5_formation [19] NAGY J B, BODART P, COLLECTTE H, FERNANDEZ C, GABELICA Z, NASTRO A, AIELLO R. Characterization of crystalline and amorphous phases during the synthesis of (TPA, M)-ZSM-5 zeolites (M=Li, Na, K)[J]. J Chem Soc, Faraday Trans, 1989, 185: 2749-2769. [20] DAI C Y, ZHANG A F, LI L L, HOU K K, DING F S, LI J, MU D Y, SONG C S, LIU M, GUO X W. Synthesis of hollow nanocubes and macroporous monoliths of silicalite-1 by alkaline treatment[J]. Chem Mater, 2013, 25(21): 4197-4205. doi: 10.1021/cm401739e [21] YAN Y, CHAUDHURI S R, SARKAR A. Synthesis of oriented zeolite molecular sieve films with controlled morphologies[J]. Chem Mater, 1996, 8: 473-479. doi: 10.1021/cm950393e [22] JUNG J S, PARK J W, SEO G. Catalytic cracking of n-octane over alkali-treated MFI zeolite[J]. Appl Catal A: Gen, 2005, 288(1/2): 149-157. [23] GROEN J C, PÉREZ-RAMÍREZ J. Critical appraisal of mesopore characterization by adsorption analysis[J]. Appl Catal A: Gen, 2004, 268(1/2): 121-125. https://www.researchgate.net/publication/223238337_Critical_Appraisal_of_Mesopore_Characterization_by_Adsorption_Analysis?_sg=AIZOSND2lq0o4WG0wHWMTpDdE-L_-RBdv3Prir-8UUtURWrKzGxCNyhtMNXkzt7Vu-9Csvlenk_CItExRbGWtQ [24] LV Y Y, QIAN X F, TU B, ZHAO D. Generalized synthesis of core-shell structured nano-zeolite@ordered mesoporous silica composites[J]. Catal Today, 2013, 204: 2-7. doi: 10.1016/j.cattod.2012.09.031 [25] ARMAROLI T, SIMON L J, DIGNE M, MANTANMIA T, BEVILACQUA M, VALTCHEV V, PATARIN J, BUSCA G. Effects of crystal size and Si/Al ratio on the surface properties of H-ZSM-5 zeolites[J]. Appl Catal A: Gen, 2006, 306(1): 78-84. https://www.researchgate.net/publication/229144426_Effects_of_crystal_size_and_SiAl_ratio_on_the_surface_properties_of_H-ZSM-5_zeolites [26] 黄风林, 向小凤.甲醇精馏过程四塔流程模拟分析[J].石油与天然气化工, 2007, 36(1): 18-21. http://www.cnki.com.cn/Article/CJFDTOTAL-STQG200701007.htmHUANG Feng-lin, XIANG Xiao-feng. Simulation analysis of four-column process flow for methanol rectification[J]. Chem Eng Oil Gas, 2007, 36(1): 18-21. http://www.cnki.com.cn/Article/CJFDTOTAL-STQG200701007.htm [27] TRAN T M, GNEP S N, SZABO G, GUISNET M. Comparative study of the transformation of n-butane, n-hexane and n-heptane over H-MOR zeolites with various Si/Al ratios[J]. Appl Catal, 1998, 170(1): 49-58. doi: 10.1016/S0926-860X(98)00035-0 [28] 孔德金, 邹薇, 郑均林, 房鼎业. MFI/MFI核壳分子筛合成的影响因素及结晶动力学[J].物理化学学报, 2009, 25(9): 1921-1927. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX200909037.htmKONG De-jin, ZOU Wei, ZHENG Jun-lin, FANG Ding-ye. Crystallization kinetics and influencing factors in the syntheses of MFI/MFI core-shell zeolites[J]. Acta Phys Chim Sin, 2009, 25(9): 1921-1927. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX200909037.htm -





 下载:
下载: