Effects of paper mill residual additives on sintering and melting characteristic of wheat straw
-
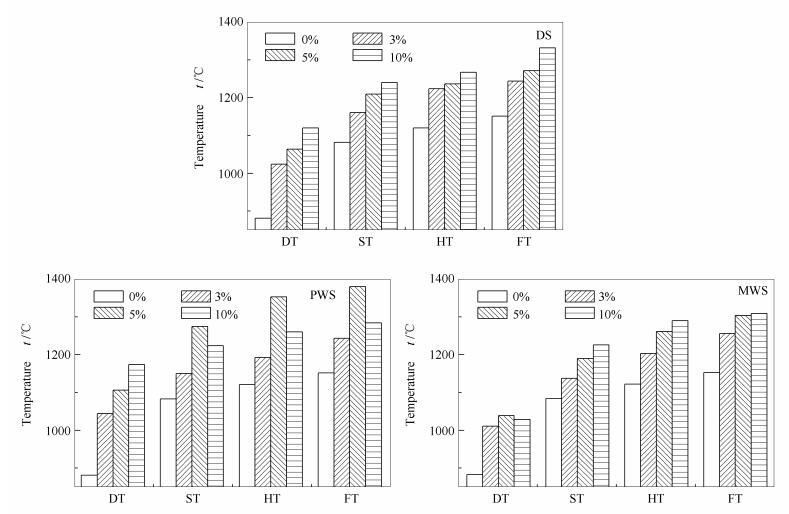
摘要: 利用灰熔点测试仪、XRD及XRF等仪器,对比研究了造纸污泥(脱墨污泥、造纸废水污泥)、城市废水污泥作为添加剂对麦秆灰熔融特性的影响,考察了烧结和熔融过程中的组分变化,分析了污泥添加剂对麦秆灰的作用机理;进一步将污泥添加剂与常规添加剂进行灰熔融特性对比研究。研究发现,添加比例控制为3%-10%,造纸污泥(脱墨污泥、造纸废水污泥)软化温度提升效果均优于城市废水污泥;在添加比例控制为5%时,造纸废水污泥对麦秆灰软化温度提升效果最好;增大添加比例过程中,造纸废水污泥Al2O3修饰骨架作用明显,但灰中长石类物质逐渐增多使得软化温度提升效果下降;在不同温度下,脱墨污泥主要是通过形成硅铝榴石使得灰熔点提升,造纸废水污泥则主要是通过生成高熔点物质CaSiO3抑制低熔点硅酸盐形成,城市废水污泥升温中存在明显SiO2晶态转变过程;使用污泥添加剂作为抗结渣添加剂具有良好应用前景。Abstract: The effects of paper mill residues (delinking sludge, paper mill sludge, municipal sewage sludge) addition on slagging tendency of wheat straw were investigated using ash melting point test system, X-ray fluorescence (XRF) and powder X-ray diffraction (XRD). The differences between other 4 common additives were also discussed. The results show that addition of delinking sludge and paper mill sludge behaves better than municipal sewage sludge in blending range of 3% to 10%, while softening temperature of wheat ash tends to get the highest value when adding 5% paper mill sludge. Along with the blends increasing, Al2O3 plays an important framework structure modification role while adding paper mill sludge. While generation of feldspar such as orthoclase and anorthite takes place, the softening temperature decreases. In addition, silicon aluminum garnet is more likely to form to enhance the fusion characteristic temperate while adding delinking sludge. On the contrary, formation of high melting point material(CaSiO3)inhibits low melting silicate by adding paper mill sludge. Components analyses indicate that crystal structure of SiO2 is changed along with municipal sludge added. Sludge used as anti-slagging additives is promising.
-
图 2 不同添加比例条件下污泥混灰的XRD谱图
Figure 2 XRD analysis of sludge mixed ash with different blendings
(a): WS-DS 1: KCl(28.4°); 2: AlPO4(26.6°); 3: KNO2(26.9°); 4: K2SO4(30.3°); 5: CaSO4(25.4°); 6: CaMgSi2O6(30.9°); 7: CaSiO3(30.0°); 8: CaMg(CO3)2(30.9°); 9: SiO2(ceosite)(28.9°); 10: Ca2MgSi2O7(31.2°); 11: KH2PO4(23.9°); 12: KNO3(29.4°) (b): WS-PWS 1: KCl(28.4°); 2: SiO2(quartz)(26.7°); 3: CaSiO3(30.0°); 4: CaMg(CO3)2(30.9°); 5: KNO2(26.9°); 6: K2MgSiO4(32.6°); 7: Ca2SiO4(32.9°); 8: SiO2(ceosite)(28.9°); 9: KAlSi3O8(26.7°); 10: Fe2SiO4(36.6°); 11: CaAl2Si2O8(28.6°); 12: AlPO4(21.0°); 13: KNO3(29.4°); 14: K2SO4(30.3°); 15: KHCO3(31.7°); 16: KPO3(26.1°); 17: KH2PO4(23.9°); 18: CaCO3(26.2°) (c): WS-MWS 1: KCl(28.4°); 2: SiO2(quartz)(26.7°); 3: AlPO4(26.6°); 4: KFe2(PO4)2(28.8°); 5: KAl3(OH)6(SO4)2(30.0°); 6: K2CO3(31.6°); 7: SiO2(cristobalite)(21.8°); 8: SiO2(ceosite)(28.9°); 9: MgSiP2(27.5°); 10: Ca3Al2Si3O12(34.7°); 11: KNO3(29.4°); 12: KAlSi2O6(27.3°); 13: K2Ca(SO4)3(27.3°); 14: Ca3Si3O9(27.1°)
图 3 麦秆特征考察温度下污泥混灰的XRD谱图
Figure 3 XRD analysis of sludge mixed ash at characteristic temperatures of wheat ash
(a): 1: KCl(28.4°); 2: SiO2(quartz)(26.7°); 3: SiO2(tridymite)(29.9°); 4: KNO3(29.4°); 5: K2SO4(30.3°); 6: KClO3(26.1°); 7: Ca3Al2Si3O12(34.3°); 8: Ca3Mg(SiO4)2(33.6°); 9: AlPO4(26.7°); 10: KAlSiO4(21.9°); 11: SiO2(20.9°); 12: Ca3SiO5(31.3°); 13: KAlSiO8(21.4°); 14: CaAl2Si2O8(26.8°); 15: CaAl2O4(35.6°) (b): 1: KCl(28.4°); 2: SiO2(quartz)(26.7°); 3: SiO2(cristobalite)(21.8°); 4: KNO3(29.4°); 5: K2Ca(CO3)2(27.9°); 6: K3P(31.5°); 7: KAlSi2O6(26.3°); 8: K2SO4(30.3°); 9: KPO3(25.6°); 10: CaMgSi(33.3°); 11: CaSiO3(30.0°); 12: Ca2MgSi2O7(31.2°); 13: Mg2SiO4(36.3°); 14: K2Si2O5(31.8°); 15: KH2PO4(23.9°); 16: SiO2(coesite)(28.9°); 17: KAlSi2O6(27.3°); 18: AlPO4(21.0°); 19: CaAl2Si2O8(26.8°); 20: CaAl2O4(35.6°); 21: Ca2SiO4(32.4°)
表 1 原料的元素分析及工业分析
Table 1 Ultimate and proximate analysis of wheat straw and paper mill residues
Sample Ultimate analysis wd/% Proximate analysis wd/% C H N S O* V FC A WS 41.63 5.84 0.92 0.17 41.49 70.95 19.1 9.95 DS 20.48 1.86 0.23 0.08 25.11 45.15 2.61 52.24 PWS 19.99 2.52 0.93 0.21 21.14 38.25 6.54 55.21 MWS 24.88 3.32 3.97 0.73 9.95 38.15 4.7 57.15 *: by difference
WS: Wheat Straw; DS: Deinking Sludge; PWS: Papermaking Wastewater Sludge;MWS: Municipal Wastewater Sludge表 2 麦秆灰及污泥灰的XRF和XRD分析
Table 2 XRF and XRD analysis results of wheat straw ash and sludge ash
WS DS PWS MWS CaO /% 7.48 76.99 57.26 5.24 Al2O3/% 0.67 6.52 19.48 22.34 SiO2 /% 52.95 11.92 13.83 42.40 MgO /% 3.98 1.81 1.64 2.53 TiO2 /% 0.05 0.85 0.51 0.81 SO3 /% 4.38 0.44 1.51 1.27 Fe2O3 /% 0.6 0.62 0.55 8.04 Na2O /% 0.61 0.49 0.54 0.33 P2O5 /% 2.14 0.14 4.39 14.56 K2O /% 19.09 0.11 0.20 2.45 Cl /% 8.05 0.09 0.09 0.01 Main
crystalline phaseKCl, SiO2(quartz),
KAlSi3O8,
K2SO4CaCO3, SiO2(quartz),
MgSiO4,
CaAl2(SiO4)2,
Ca2Al2SiO7,
K2CaP2O7CaCO3,
(Mg0.64Ca0.936)(CO3),
MgSiO3, Fe3PO7,
Ca2Al2SiO7,
K2CaP2O7SiO2(quartz),
AlSi3O8,
MgFeSiO4, CaAl4O7,
Fe4(PO4)2(OH)6·xH2O表 3 麦秆特征温度下污泥混灰组成成分分析
Table 3 Composition of mixed ashes at characteristic temperatures of wheat ash
Content w/% t/℃ CaO Al2O3 SiO2 MgO TiO2 SO3 Fe2O3 Na2O P2O5 K2O Cl WS ash 835 7.87 0.81 55.88 3.07 0.07 0.88 1.08 0.59 1.88 19.91 7.94 990 8.59 0.85 53.31 3.10 0.07 1.47 1.33 0.67 1.59 19.83 9.20 DS ash 835 32.74 5.85 37.84 2.41 0.36 2.85 1.06 0.56 3.56 9.89 2.97 990 39.96 3.76 34.50 2.98 0.41 2.33 0.76 0.48 1.56 9.63 3.61 PWS ash 835 28.65 9.46 37.63 2.35 0.26 3.06 1.00 0.54 3.45 10.34 3.25 990 32.94 10.15 34.88 2.96 0.31 0.88 0.77 0.42 3.75 9.44 3.49 MWS ash 835 4.16 1.21 34.48 1.40 0.07 0.11 1.04 0.32 47.09 9.99 0.12 990 6.81 10.77 54.03 2.66 0.42 0.28 4.31 0.50 7.85 10.99 1.37 -
[1] WANG L, SKREIBERG O, BECIDAN M, LI H L. Investigation of rye straw ash sintering characteristics and the effect of additives[J]. Appl Energy, 2016, 162:1195-1204. doi: 10.1016/j.apenergy.2015.05.027 [2] WANG L, BECIDAN M, SKREIBERG O. Sintering behavior of agricultural residues ashes and effects of additives[J]. Energy Fuels, 2012, 26(9):5917-5929. doi: 10.1021/ef3004366 [3] STEENARI B M, LINDQVIST O. High-temperature reactions of straw ash and the anti-sintering additives kaolin and dolomite[J]. Biomass Bioenergy, 1998, 14(1):67-76. doi: 10.1016/S0961-9534(97)00035-4 [4] OHMAN M, NORDIN A, LUNDHOLM K, BOSTROM D, HEDMAN H, LUNDBERG M. Ash transformations during combustion of meat-, bonemeal, and RDF in a (bench-scale) fluidized bed combustor[J]. Energy Fuels, 2003, 17(5):1153-1159. doi: 10.1021/ef020273a [5] QI J H, LI H, HAN K H, ZUO Q, GAO J, WANG Q, LU C M. Influence of ammonium dihydrogen phosphate on potassium retention and ash melting characteristics during combustion of biomass[J]. Energy, 2016, 102:244-251. doi: 10.1016/j.energy.2016.02.090 [6] GRIMM A, SKOGLUND N, BOSTROM D, BOMAN C, OHMAN M. Influence of phosphorus on alkali distribution during combustion of logging residues and wheat straw in a bench-scale fluidized bed[J]. Energy Fuels, 2012, 26(5):3012-3023. doi: 10.1021/ef300275e [7] LI H, HAN K H, WANG Q, LU C M. Influence of ammonium phosphates on gaseous potassium release and ash-forming characteristics during combustion of biomass[J]. Energy Fuels, 2015, 29(4):2555-63. doi: 10.1021/acs.energyfuels.5b00285 [8] 夏海渊, 袁洪友, 王贵金, 周肇秋, 苏德仁, 杨卿, 阴秀丽.脱墨污泥热解气化特性研究[J].造纸科学与技术, 2012, 3:88-92.XIA Han-yuan, YUAN Hong-you, WANG Gui-jin, ZHOU Zhao-qiu, SU De-ren, YANG Qing, YIN Xiu-li. Experimental study on pyrolysis and gasification characteristics of deinking sludge[J]. Paper Sci Technol, 2012, 3:88-92. [9] FERREIRA C I A, CALISTO V, CUERDA-CORREA E M, OTERO M, NADAIS H, ESTEVES V I. Comparative valorisation of agricultural and industrial biowastes by combustion and pyrolysis[J]. Bioresour Technol, 2016, 218:918-925. doi: 10.1016/j.biortech.2016.07.047 [10] SKOGLUND N, GRIMM A, OHMAN M, BOSTROM D. Effects on ash chemistry when co-firing municipal sewage sludge and wheat straw in a fluidized bed:Influence on the ash chemistry by fuel mixing[J]. Energy Fuels, 2013, 27(10):5725-5732. doi: 10.1021/ef401197q [11] WANG L, SKJEVRAK G, HUSTAD J E, GRONLI M G. Effects of sewage sludge and marble sludge addition on slag characteristics during wood waste pellets combustion[J]. Energy Fuels, 2011, 25(12):5775-5785. doi: 10.1021/ef2007722 [12] DAVIDSSON K O, AMAND L E, ELLED A L, LECKNER B. Effect of cofiring coal and biofuel with sewage sludge on alkali problems in a circulating fluidized bed boiler[J]. Energy Fuels, 2007, 21(6):3180-3188. doi: 10.1021/ef700384c [13] HUPA M. Ash-related issues in fluidized-bed combustion of biomasses:recent research highlights[J]. Energy Fuels, 2012, 26(1):4-14. doi: 10.1021/ef201169k [14] BROSTROM M, KASSMAN H, HELGESSON A, BERG M, ANDERSSON C, BACKMAN R, NORDIN A. Sulfation of corrosive alkali chlorides by ammonium sulfate in a biomass fired CFB boiler[J]. Fuel Process Technol, 2007, 88(11/12):1171-1177. [15] LI L N, REN Q Q, LI S Y, LU Q G. Effect of phosphorus on the behavior of potassium during the co-combustion of wheat straw with municipal sewage sludge[J]. Energ Fuels, 2013, 27(10):5923-5930. doi: 10.1021/ef401196y [16] ARING, MAND L E, LECKNER B, ESKILSSON D, TULLIN C. Deposits on heat transfer tubes during co-combustion of biofuels and sewage sludge[J]. Fuel, 2006, 85(10/11):1313-1322. [17] NIU Y Q, TAN H. Z, HUI S E. Ash-related issues during biomass combustion:Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures[J]. Prog Energy Combust Sci, 2016, 52:1-61. doi: 10.1016/j.pecs.2015.09.003 [18] GILBE C, LINDSTROM E, BACKMAN R, SAMUELSSON R, BURVALL J, OHMAN M. Predicting slagging tendencies for biomass pellets fired in residential appliances:A comparison of different prediction methods[J]. Energy Fuels, 2008, 22(6):3680-3686. doi: 10.1021/ef800321h [19] WANG S, JIANG X M, HAN X X, WANG H. Fusion characteristic study on seaweed biomass ash[J]. Energy Fuels, 2008, 22(4):2229-2235. doi: 10.1021/ef800128k [20] 牛艳青, 谭厚章, 王学斌, 徐通模, 刘正宁, 刘洋.辣椒秆灰熔融特性分析[J].中国电机工程学报, 2010, 11:68-72. http://d.wanfangdata.com.cn/Thesis/D625770NIU Yan-qing, TAN Hou-zhang, WANG Xue-bin, XU Tong-mo, LIU Zheng-ning, LIU Yang. Fusion characteristic of capsicum stalks ash[J]. Proc CSEE, 2011, 11:68-72. http://d.wanfangdata.com.cn/Thesis/D625770 [21] PRIYANTO D E, UENO S, SATO N, KASAI H, TANOUE T, FUKUSHIMA H. Ash transformation by co-firing of coal with high ratios of woody biomass and effect on slagging propensity[J]. Fuel, 2016, 174:172-179. doi: 10.1016/j.fuel.2016.01.072 [22] MA T, FAN C G, HAO L F, LI S G, SONG W L, LI W G. Biomass-ash-induced agglomeration in a fluidized bed. Part 1:Experimental study on the effects of a gas atmosphere[J]. Energy Fuels, 2016, 30(8):6395-6404. doi: 10.1021/acs.energyfuels.6b00164 [23] 李婷婷, 黄艳琴, 袁宏友, 刘华财, 袁洪友, 阴秀丽, 吴创之.基于电容测试方法的麦秆灰、烧结熔融特性研究[J].太阳能学报, 2018, 34.LI Ting-ting, HUANG Yan-qin, YUAN Hong-you, LIU Hua-cai, YIN Xiu-li, WU Chuang-zhi. Characterization of sintering behavior of wheat straw ash based on capacitance test[J]. Acta Energi Sina, 2018, 34. [24] 李文, 白进.煤的灰化学[M].北京:科学出版社, 2013, 84.LI Wen, BAI Jin. Chemistry of Ash from Coal[M]. Beijing:Science Press, 2013, 84. [25] WANG, L, SKJEVRAK G, HUSTAD J E, GRONLI M G. Sintering characteristics of sewage sludge ashes at elevated temperatures[J]. Fuel Process Technol, 2012, 96:88-97. doi: 10.1016/j.fuproc.2011.12.022 [26] BLÄSING M, ZINI M, MVLLER M. Influence of feedstock on the release of potassium, sodium, chlorine, sulfur, and phosphorus species during gasification of wood and biomass shells[J]. Energy Fuels, 2013, 27(3):1439-1445. doi: 10.1021/ef302093r [27] WU H, CASTRO M, JENSEN P A, FRANDSEN F J, GLARBORG PETER DAM-JOHANSEN K, RØKKE M, LUNDTORP K. Release and transformation of inorganic elements in combustion of a high-phosphorus fuel[J]. Energy Fuels, 2011, 25(7):2874-2886. doi: 10.1021/ef200454y [28] 袁艳文, 赵立欣, 孟海波, 林聪, 田宜水.玉米秸秆颗粒燃料抗结渣剂效果的比较[J].农业工程学报, 2010, 11:251-255. doi: 10.3969/j.issn.1002-6819.2010.09.041YUAN Yan-wen, ZHAO Li-xin, MENG Hai-bo, LIN Chong, TIAN Yi-shui. Effects comparison on anti-slagging additives of corn straw biomass pellet fuel[J].Trans CSAE, 2010, 11:251-255. doi: 10.3969/j.issn.1002-6819.2010.09.041 [29] LI L N, REN Q Q, LI S Y, LU Q G. Effect of phosphorus on the behavior of potassium during the Co-combustion of wheat straw with municipal sewage sludge[J]. Energy Fuels, 2013, 27(10):5923-5930. doi: 10.1021/ef401196y [30] LI H, HAN K H, WANG Q, LU C M. Pyrolysis of rice straw with ammonium dihydrogen phosphate:Properties and gaseous potassium release characteristics during combustion of the products[J]. Bioresour Technol, 2015, 197:193-200. doi: 10.1016/j.biortech.2015.08.070 -





 下载:
下载: