Preparation and deep adsorption denitrification from diesel oil of heteroatoms mesoporous molecular sieve Co-MCM-41
-
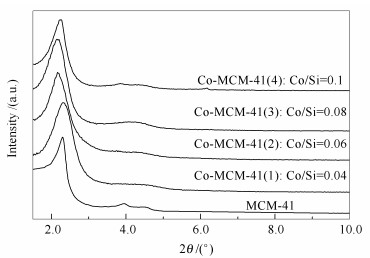
摘要: 以硝酸钴为钴源, 采用水热法合成了MCM-41和不同Co含量的Co-MCM-41分子筛, 并利用XRD、FT-IR和低温N2吸附-脱附等方法对合成的分子筛进行表征。当加入的Co/Si物质的量比达到0.1时, 依然能够成功合成具有规整有序的介孔结构的Co-MCM-41。MCM-41和Co-MCM-41静态吸附脱除0#柴油中碱氮的实验结果表明, Co/Si物质的量比为0.06的Co-MCM-41(2) 分子筛的吸附容量最大, 达到5.324 mg (N)/g分子筛, 明显高于MCM-41分子筛的吸附容量2.532 mg (N)/g, 说明Co进入MCM-41分子筛骨架后显著提高了分子筛的吸附脱除碱氮能力。当加入的Co/Si物质的量比大于0.06时, 分子筛吸附脱除柴油中碱氮的能力反而下降, 这是由于加入过多Co会使其以Co3O4形式高度分散在分子筛孔道中, 堵塞了吸附活性位, 使其无法与碱性氮化物接触造成吸附脱氮能力下降。动态吸附脱除0#柴油中碱性氮化物的结果表明, 每克Co-MCM-41(2) 分子筛可将35 mL柴油的碱氮从147.54 μg/g吸附脱除到10 μg/g以下, 吸附容量为4.2 mg (N)/g (吸附剂), 由于动态吸附的接触时间较短使MCM-41失去了吸附脱氮能力, 说明Co-MCM-41(2) 对柴油中的碱氮具有较好的选择性。Abstract: The mesoporous molecular sieves MCM-41 and Co-MCM-41 containing different cobalt contents were prepared by hydrothermal synthesis method with cobalt nitrate as cobalt source and characterized using X-ray diffraction (XRD), Fourier transform infrared spectrum (FT-IR) and nitrogen adsorption. The characterization results indicate that the well-ordered mesostructure was obtained even when the Co/Si mol ratio was 0.1 and Co has been introduced into the framework of MCM-41. Adsorption removal of basic nitrogen compounds from 0# diesel oil was studied by static stirring method using MCM-41 and Co-MCM-41. One gram of Co-MCM-41(2) could adsorb 5.324 mg nitrogen from diesel oil while MCM-41 could adsorb 2.532 mg, indicating that the adsorptive denitrification of Co-MCM-41(2) has been enhanced pronouncedly. But the adsorptive denitrification capacity of Co-MCM-41(2) decreased when the Co/Si exceeded 0.06, which could be ascribed to the addition of exceeded Co into the frameworks of MCM-41. The Co was well dispersed in the channel of Co-MCM-41 as Co3O4 and blocked the adsorption active sites which hindered the adsorption between the nitrogen compounds and active sites and depressed the adsorptive denitrification capacity. The results of adsorptive denitrification from diesel oil using dynamic adsorption method showed that for a breakthrough point of 10 μg/g, the breakthrough volume and breakthrough capacity of Co-MCM-41(2) at ambient conditions are 35 mL/g-adsorbent and 4.2 mg-nitrogen/g adsorbent, respectively. For MCM-41, both of the two data were almost zero, indicating that MCM-41 almost lost all the adsorptive denitrification capacity due to the shorter contact time and implying that Co-MCM-41(2) had better selectivity on basic nitrogen compounds in 0# diesel oil.
-
Key words:
- Co-MCM-41 /
- synthesis /
- chatacterization /
- diesel oil /
- denitrification
-
图 5 MCM-41和Co-MCM-41吸附脱除柴油中碱氮的剩余含量、碱氮脱除率及氮吸附容量
Figure 5 Absorption capacity, basic nitrogen removal rate and remained basic nitrogen content of 0# diesel oil treated by MCM-41 or Co-MCM-41 (static stirring method; ambient conditions; 25 mL 0# diesel oil was treated with 0.5 g molecular sieve)
: remained basic nitrogen content; : adsorption capacity; : removal rate
表 1 MCM-41和Co-MCM-41的孔结构参数
Table 1 Pore structure parameters of MCM-41 and Co-MCM-41 samples
Initiator Value MCM-41 Co-MCM-41(1) Co-MCM-41(2) Co-MCM-41(3) Co-MCM-41(4) 2θ/(°)(100) 2.308 2 2.305 0 2.180 4 2.164 8 2.220 3 d100/nm 3.823 0 3.828 3 4.047 0 4.076 2 3.974 3 a0/nm 4.414 5 4.420 6 4.673 2 4.706 9 4.589 2 Total pore volume v/(cm3·g-1) 0.887 5 0.785 3 0.787 2 0.838 9 0.745 2 As, BET/(m2·g-1) 983.04 836.44 868.20 889.53 812.55 Average pore diameter d/nm 3.11 3.21 3.22 3.27 3.16 note:$2{d_{100}}\sin \theta = n\lambda ,{\alpha _0} = \frac{{2{d_{100}}}}{{\sqrt 3 }}$ -
[1] DHEER S, ANJU C, MITRA B P, AMARJIT S S. A comparative evaluation of nitrogen compounds in petroleum distillates[J]. Chromatographia, 2011, 74(1/2): 121-126. https://www.researchgate.net/publication/257495567_A_Comparative_Evaluation_of_Nitrogen_Compounds_in_Petroleum_Distillates [2] JVRGEN B, JVRGEN K, ANDREAS W O S, THOMAS B, MICHAEL M E H, GÖTZ W. Influence of fuel properties, nitrogen oxides, and exhaust treatment by an oxidation catalytic converter on the mutagenicity of diesel engine emissions[J]. Arch Toxicol, 2006, 80(8): 540-546. doi: 10.1007/s00204-006-0088-y [3] LIU K, FLORA T T. Effect of the nitrogen heterocyclic compounds on hydrodesulfurization using in situ hydrogen and a dispersed Mo catalyst[J]. Catal Today, 2010, 149(1/2): 28-34. https://www.researchgate.net/publication/244322858_Effect_of_the_nitrogen_heterocyclic_compounds_on_hydrodesulfurization_using_in_situ_hydrogen_and_a_dispersed_Mo_catalyst [4] HANAFI S A, MOHAMED M S. Recent trends in the cleaning of diesel fuels via desulfurization processes[J]. Energy Sources Part A, 2011, 33(6): 495-511. doi: 10.1080/15567030903058360 [5] 朱金柱, 沈健. SBA-15吸附脱除油品中的碱性氮化物[J].石油学报(石油加工), 2012, 40(11): 566-570. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG201204008.htmZHU Jin-zhu, SHEN Jian. Adsorption of basic nitrogen compounds from oil by SBA-15 zeolite[J]. Acta Pet Sin (Pet Process Sect), 2012, 40(11): 566-570. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG201204008.htm [6] 朱金柱, 沈健, 韩英. Nb-SBA-15的制备及吸附脱氮性能[J].硅酸盐学报, 2012, 40(11): 1666-1670. http://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201211020.htmZHU Jin-zhu, SHEN Jian, HAN Ying. Preparation and adsoparation denitrication of Nb-SBA-15 zeolite[J]. J Chin Ceram Soc, 2012, 40(11): 1666-1670. http://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201211020.htm [7] 洪新, 唐克. NaY分子筛的改性及吸附脱氮性能[J].燃料化学学报, 2015, 43(2): 1-7. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18577.shtmlHONG Xin, TANG Ke. Modification and adsorptive denitrification of NaY molecular sieve[J]. J Fuel Chem Technol, 2015, 43(2): 1-7. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18577.shtml [8] 翟玉龙, 沈健. HY分子筛吸附脱除油品中碱性氮化物的研究[J].石油炼制与化工, 2011, 42(1): 41-44. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201101017.htmZHAI Yu-long, SHEN Jian. Study on the adsorption of basic nitrogen compounds from oil with HY molecular sieve[J]. Pet Process Petrochem, 2011, 42(1): 41-44. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201101017.htm [9] 王福帅, 李会鹏, 赵华, 房斌斌. NaY/β复合分子筛改性及对模拟柴油中氮化物的吸附性能[J].石油炼制与化工, 2012, 43(11): 59-62. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201211016.htmWANG Fu-shuai, LI Hui-peng, ZHAO Hua, FANG Bin-bin. Modification of NaY/β composite molecular sieve and its adsorption performance of removing nitrogen containing compounds in model oil[J]. Pet Process Petrochem, 2012, 43(11): 59-62. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201211016.htm [10] 王云芳, 迟志明.介孔分子筛Al-MCM-41的吸附脱氮性能研究[J].石化技术与应用, 2014, 32(2): 113-117. http://www.cnki.com.cn/Article/CJFDTOTAL-IZHM201402003.htmWANG Yun-fang, CHI Zhi-ming. Research on nitrogen adsorption removal performance of mesoporous molecular sieve Al-MCM-41[J]. Petrochem Technol Appl, 2014, 32(2): 113-117. http://www.cnki.com.cn/Article/CJFDTOTAL-IZHM201402003.htm [11] 迟志明.介孔分子筛用于柴油吸附脱氮的基础研究[D].北京:中国石油大学, 2010.CHI Zhi-ming. Basic research of Al-MCM-41 molecular sieve for denitrogenation of diese oil[D]. Beijing: China University of Petroleum, 2010. [12] 张晗.燃料中含氮化合物的吸附脱除[D].大连:大连理工大学, 2009.ZHANG Han. Adsorptive removal of nitrogen-containing compounds from fuel[D]. Dalian: Dalian University of Technology, 2009. [13] 洪新, 唐克.杂原子介孔Co-MCM-41分子筛的制备及其吸附脱氮性能[J].燃料化学学报, 2015, 43(6): 720-727. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18646.shtmlHONG Xin, TANG Ke. Preparation and adsorption denitrification of heteroatoms mesoporous molecular sieve Co-MCM-41[J]. J Fuel Chem Technol, 2015, 43(6): 720-727. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18646.shtml [14] LUAN Z H, XU J, HE H Y, JACEK K, LARRY K. Synthesis and spectroscopic characterization of vanadosilicate mesoporous MCM-41 molecular sieves[J]. J Phys Chem, 1996, 100(50): 19595-1960. doi: 10.1021/jp962353j [15] 李亚男.掺杂过渡金属的MCM-41介孔分分子筛的制备、表征及其对CO2乙烷氧化脱氢制乙烯的研究[D].长春:吉林大学, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10183-2006110555.htmLI Ya-nan. Synthesis, characterization and catalytic activity of Me-MCM-41 for oxidative dehydrogenation of ethane to ethylene with CO2[D]. Changchun: Jilin University, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10183-2006110555.htm [16] PARVULESCU V, SU B L. Iron, cobalt or nickel substituted MCM-41 molecular sieves for oxidation of hydrocarbons[J]. Catal Today, 2001, 69(1/4): 315-322. https://www.researchgate.net/publication/223779525_Iron_cobalt_or_nickel_substituted_MCM-41_molecular_sieves_for_oxidation_of_hydrocarbons [17] ARAUJO A S, JARONIEC M. Synthesis and properties of Lanthanide incorporated mesoporous molecular sieves[J]. J Colloid Interface Sci, 1999, 218(2): 462-467. doi: 10.1006/jcis.1999.6437 [18] ZHAO Q, WANG Q, TANG Y J, JIANG T S, LI CS, YIN H B. Characterization and synthesis of Ce-incorporated mesoporous molecular sieves under microwave irradiation condition[J]. Korean J Chem Eng, 2010, 27(4): 1310-1315. doi: 10.1007/s11814-010-0182-y [19] 赵谦, 胡晓笑, 张蓉仙, 李梅, 姜廷顺. Co-MCM-41介孔分子筛的水热合成与稳定性[J].中国有色金属学报, 2009, 19(1): 189-194. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ200901029.htmZHAO Qian, HU Xiao-xiao, ZHANG Rong-xian, LI Mei, JIANG Ting-shun. Stability and hydrothermal synthesis of Co-MCM-41 mesoporous molecular sieves[J]. Chin J Nonferrous Met, 2009, 19(1): 189-194. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ200901029.htm [20] 张燕, 李湘祁, 陈琼霞, 汤德平.微波法合成有序Co-MCM-41介孔分子筛[J].化工时刊, 2008, 22(2): 12-15. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJS200802006.htmZHANG Yan, LI Xiang-qi, CHEN Qiong-xia, TANG De-ping. Microwave synthesis of ordered mesoporous Co-MCM-41[J]. Chem Ind Times, 2008, 22(2): 12-15. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJS200802006.htm [21] JIANG T S, SHEN W, ZHAO Q, LI M, CHU J Y, YIN H B. Characterization of CoMCM-41 mesoporous molecular sieves obtained by the microwave irradiation method[J]. J Solid State Chem, 2008, 181(9): 2298-2305. doi: 10.1016/j.jssc.2008.05.010 [22] 李亚男, 郭晓红, 周广栋, 毕颖丽, 李文兴, 程铁欣, 吴通好, 甄开吉. Co-MCM-41催化剂上临CO2-乙烷脱氢反应的研究[J].高等学校化学学报, 2005, 26(6): 1122-1125. http://www.cqvip.com/Main/Detail.aspx?id=15749250LI Ya-nan, GUO Xiao-dong, ZHOU Guang-dong, BI Ying-li, LI Wen-xing, CHENG Tie-xin, WU Tong-hao, ZHEN Kai-ji. CO2-dehydrogenation of ethane over Co-MCM-41 catalyst[J]. Chem J Chin Univ, 2005, 26(6): 1122-1125. http://www.cqvip.com/Main/Detail.aspx?id=15749250 [23] 于健强, 李灿, 许磊, 李美俊, 辛勤, 刘中民.以硅溶胶和三氯化钛为原料合成Ti-MCM-41分子筛Ⅱ.Ti-MCM-41分子筛的表征[J].催化学报, 2001, 22(4): 331-334. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA200104004.htmYU Jian-qiang, LI Can, XU L, LI Mei-jun, XIN Qin, LIU Zhong-min. Synthesis of Ti-MCM-41 using colloidal silica and titanium trichloride Ⅱ.Characterization of Ti-MCM-41 molecular sieve[J]. Chin J Catal, 2001, 22(4): 331-334. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA200104004.htm [24] 赵杉林, 张扬健, 孙桂大, 翟玉春.钒硅MCM-41沸石分子筛微波合成与表征[J].燃料化学学报, 1999, 27(2): 130-133. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX902.006.htmZHAO Shan-lin, ZHANG Yang-jian, SUN Gui-da, ZHAI Yu-chun. Synthesis of mesoporous molecular sieve vMCM-41 by microwave radiation and its characterization[J]. J Fuel Chem Technol, 1999, 27(2): 130-133. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX902.006.htm [25] ZHENG J, CHU W, ZHANG H, JIANG C F, DAI X Y. CO oxidation over Co3O4/SiO2 catalysts: Effects of porous structure of silica and catalyst calcination temperature[J]. J Nat Gas Chem, 2010, 19(6): 583-588. doi: 10.1016/S1003-9953(09)60119-5 [26] VARGHESE S, CUTRUFELLO M G, ROMBI E, CANNAS C, MONACI R, FERINO I. CO oxidation and preferential oxidation of CO in the presence of hydrogen over SBA-15-templated CuO-Co3O4 catalysts[J]. Appl Catal A: Gen, 2012, 443-444: 161-170. doi: 10.1016/j.apcata.2012.07.038 [27] 刘艳. Co3O4基负极材料的制备、表征及性能研究[D].南京:南京航空航天大学, 2009.LIU Yan. Synthesis, characterization and properties of Co3O4-based anode materials[D]. Nanjing: Nanjing University of Aeronautics and Astronautics, 2009. -





 下载:
下载: