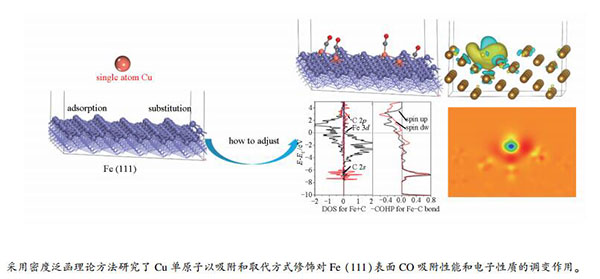
First-principles study on the CO adsorption and electronic properties of Fe (111) modified by Cu single atom
-
摘要: 采用密度泛函理论方法研究了Cu单原子修饰对Fe(111)表面CO吸附性能和电子性质的调变作用,其中,Cu单原子修饰研究了吸附和取代两种方式。结果表明,CO在Cu修饰的Fe(111)面吸附能力都会变弱,一是Cu原子自身提供的位点对CO的吸附较弱;二是Cu会使其附近的Fe对CO的吸附变弱。分析电子性质表明,Cu作用于Fe表面后,会导致Cu附近Fe原子部分电子向Cu原子转移,进而削弱了Fe与吸附分子间电子交互作用而改变Fe原子的吸附能力。故Cu原子改性Fe表面可以很好地调变CO的吸附、解离及后续反应催化活性,这为进一步探究Cu改性Fe表面的合成气催化反应机理提供了基础信息。Abstract: In this paper, the effect of Cu single atom modification on the adsorption of CO and electronic properties of Fe (111) surface has been studied by density functional theory (DFT). Two ways of adsorption and substitution have been studied for Cu mono-atom modification. The results show that the adsorption capacity of CO on the Cu modified Fe (111) becomes weak. One reason is that the sites provided by the Cu atom itself are weak for CO, and the other is that Cu weakens the adsorption of CO on the Fe nearby Cu. The analysis of electronic properties indicates that when Cu acts on the Fe (111), the part electrons of Fe can be transferred to the Cu, which weakens the electronic interaction between Fe and adsorbed molecules, and adjusts its adsorption capacity. Therefore, the Fe surface modified by Cu atom can well adjust the adsorption, dissociation and subsequent reaction catalytic activity of CO, which provides basic information to further explore the syngas catalytic reaction mechanism of Cu modified Fe surface.
-
Key words:
- Fe (111) /
- density functional theory /
- monoatomic Cu modification /
- CO adsorption
-
图 1 Cu在Fe (111)面的稳定作用结构和对应能量
Figure 1 Stability of structure and the corresponding energy of Cu acted with Fe(111)
(a): Cu adsorbed on deep hole; (b): Cu adsorbed on the shallow hole; (c): Cu instead of surface Fe; (d): Cu instead of subsurface Fe (light blue for the outermost Fe, gray for subsurface Fe, dark blue for the third layer of Fe)
表 1 CO在纯Fe (111)、Cu-ads-DH和Cu-sub-OM三种不同表面吸附能及C-O键长比较
Table 1 Comparison of adsorption energy (eV) and bond length (nm) of CO on pure Fe (111) surface and Cu modified Fe (111)
Adsorption site Fe (111) Cu-ads-DH Cu-subs-OM dM-C dC-O Eads dM-C dC-O Eads dM-C dC-O Eads CO top-Cu - - - 0.1517 - -1.10 0.1526 - -1.10 top-1thFe 0.1586 - -1.95 0.1705 - -1.55 0.1584 - -1.36 (adjacent Cu) 0.1672 top-2thFe 0.1603 - -2.68 0.1760 - -1.84 0.1672 - -1.94 (adjacent Cu) 0.1806 0.1763 Fe-Fe-bri -2.52 -1.91 -2.09 表 2 纯Fe (111)、Cu-ads-DH和Cu-subs-OM三种结构表层原子Bader电荷分析
Table 2 Bader charge of surface atoms of pure Fe (111) and Cu-ads-DH and Cu-subs-OM models
Fe(111) Cu-ads-DH Cu-subs-OM Cu-Δ(e) - 0.239 0.147 1stFe-Δ(e) -0.086 -0.101 -0.032 2ndFe-Δ(e) 0.160 0.032 0.035 (1stFe+ 2ndFe) -Δ(e) 0.074 -0.069 0.003 -
[1] ZHANG Q, KANG J, WANG Y. Development of novel catalysts for Fischer-Tropsch synthesis:Tuning the product selectivity[J]. ChemCatChem, 2010, 2:1030-1058. doi: 10.1002/cctc.201000071 [2] 王润平, 毛树红, 池永庆, 段秀琴, 刘军.费托合成铁基催化剂助剂的研究概述[J].天津化工, 2008, 22:17-19. http://d.old.wanfangdata.com.cn/Periodical/tjhg200801006WANG Run-ping, MAO Shu-hong, CHI Yong-hong, DUAN Xiu-qin, LIU Jun. Overview of the study on FT synthesis of iron-based catalyst auxiliaries[J]. Tianjin Chem Ind, 2008, 22:17-19. http://d.old.wanfangdata.com.cn/Periodical/tjhg200801006 [3] HUO C F, WU B S, GAO P, YANG Y, LI Y W, JIAO H. The mechanism of potassium promoter:Enhancing the stability of active surfaces[J]. Angew Chem Int Ed, 2011, 50:7403-7406. doi: 10.1002/anie.201007484 [4] VAN STEEN E, CLAEYS M. Fischer-Tropsch catalysts for the biomass-to-liquid process[J]. Chem Eng Technol, 2008, 31:655-666. doi: 10.1002/ceat.200800067 [5] CHONCO Z H, FERREIRA A, LODYA L, CLAEYSM, VAN STEEN E. Comparing silver and copper as promoters in Fe-based Fischer-Tropsch catalysts using delafossite as a model compound[J]. J Catal, 2013, 307:283-294. doi: 10.1016/j.jcat.2013.08.005 [6] CHONCO Z H, LODYA L, CLAEYS M, VAN STEEN E. A model for investigating the role of copper in the dynamic iron-based Fischer-Tropsch catalyst[J]. J Catal, 2013, 308:363-373. doi: 10.1016/j.jcat.2013.08.012 [7] O'BRIEN R J, DAVIS B H. Impact of copper on an alkali promoted Iron Fischer-Tropsch catalyst[J]. Catal Lett, 2004, 94:1-6. doi: 10.1023/B:CATL.0000019322.69160.ef [8] 胡伟.合成气制低碳醇Cu-Fe催化剂的制备及改性机制研究[D].上海: 华东理工大学, 2017.HU Wei. Preparation Of Cu-Fe Catalysts For Low Carbon Alcohols And Mechanism Research[D]. Shanghai: East China University of Science and Technology, 2017. [9] 李明阳, 李涛.铁改性Cu/Zn/MgO催化剂对合成气制低碳醇的影响[J].精细化工, 2015, 32(6):646-651. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201506010LI Ming-yang, LI Tao. Effect of Fe modified Cu/Zn/MgO catalyst on the synthesis of lower alcohol from syngas[J]. Fine Chem, 2015, 32(6):646-651. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201506010 [10] HE S, WANG W, SHEN Z. Carbon nanotube-supported bimetallic Cu-Fe catalysts for syngas conversion to higher alcohols[J]. Mol Catal, 2019, 479:110610. doi: 10.1016/j.mcat.2019.110610 [11] SHI X, YU H, GAO S. Synergistic effect of nitrogen-doped carbon-nanotube-supported Cu-Fe catalyst for the synthesis of higher alcohols from syngas[J]. Fuel, 2017, 210:241-248. doi: 10.1016/j.fuel.2017.08.064 [12] WANG T, TIAN X X, LI Y W, WANG J, BELLER M, JIAO H. Coverage-dependent CO adsorption and dissociation mechanisms on iron surfaces from DFT computations[J]. ACS Catal, 2014, 4(6):1991-2005. doi: 10.1021/cs500287r [13] WANG T, TIAN X, YANG Y, LI Y, WANG J, BELLER M, JIAO H. Co-adsorption and mutual interaction of nCO+mH2 on the Fe(110) and Fe(111) surfaces[J]. Catal Today, 2015, 261:82-92. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7decc2de97469e87db4578743d442035 [14] LIU S, LI Y, WANG J, JIAO H. Reactions of CO, H2O, CO2, and H2 on the clean and precovered Fe(110) surfaces-A DFT investigation[J]. J Phys Chem C, 2015, 119(51):28377-28388. doi: 10.1021/acs.jpcc.5b07497 [15] CAO D B, WANG S G, LI Y W, WANG J, JIAO H. What is the product of ketene hydrogenation on Fe5C2(001):Oxygenates or hydrocarbons?[J]. J Mol Catal A:Chem, 2007, 272(1/2):275-287. [16] LING L, WANG Q, ZHANG R. Formation of C2 oxygenates and ethanol from syngas on an Fe-decorated Cu-based catalyst:Insight into the role of Fe as a promoter[J]. Phys Chem Chem Phys, 2017, 19(45):30883-30894. doi: 10.1039/C7CP05411D [17] TIAN X, WANG T, YANG Y, LI Y W, JIAO H J. Structures and energies of Cu clusters on Fe and Fe3C surfaces from density functional theory computation[J]. Phys Chem Chem Phys, 2014, 16(48):26997-27011. doi: 10.1039/C4CP04012K [18] 赵训华, 李永旺, 王建国, 霍春芳. Fe(100)表面Cu单层膜上CO的吸附解离以及C-C偶合反应[J].燃料化学学报, 2011, 39(12):956-960. doi: 10.3969/j.issn.0253-2409.2011.12.013ZHAO Xun-hua, LI Yong-wang, WANG Jian-guo, HUO Chun-fang. CO adsorption, CO dissociation, and CC coupling on Cu monolayer-covered Fe (100)[J]. J Fuel Chem Technol, 2011, 39(12):956-960. doi: 10.3969/j.issn.0253-2409.2011.12.013 [19] KRESSE G, FURTHMVLLER J. Efficiency of Ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput Mater Sci, 1996, 6:15-50. doi: 10.1016/0927-0256(96)00008-0 [20] KRESSE G, FURTHMVLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Phys Rev B, 1996, 54:11169-11186. doi: 10.1103/PhysRevB.54.11169 [21] BLÖCHL P E. Projector augmented-wave method[J]. Phys Rev B, 1994, 50:17953-17979. doi: 10.1103/PhysRevB.50.17953 [22] KRESSE G, HAFNER J. First-principles study of the adsorption of atomic H on Ni (111), (100) and (110)[J]. Surf Sci, 2000, 459:287-302. doi: 10.1016/S0039-6028(00)00457-X [23] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1996, 77:3865-3868. doi: 10.1103/PhysRevLett.77.3865 [24] PERDEW J P, BURKEK, ERNZERHOF M. ERRATA:Generalized gradient approximation made simple[J]. Phys Rev Lett, 1997, 78:1396. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_b31ae225ab43d9cfa9076b238ba448e1 [25] METHFESSEL M, PAXTON A T. High-precision sampling for brillouin-zone integration in metals[J]. Phys Rev B, 1989, 40:3616. doi: 10.1103/PhysRevB.40.3616 [26] BLIGAARD T, NØRSKOV J K, DAHL S, MATTHIESEN J, CHRISTENSEN CH, SEHESTED J. The Brønsted-Evans-Polanyi relation and the volcano curve in heterogeneous catalysis[J]. J Catal, 2004, 224:206-217. doi: 10.1016/j.jcat.2004.02.034 -





 下载:
下载: