Catalytic combustion of volatile organic compounds over CuO-CeO2 supported on SiO2-Al2O3 modified glass-fiber honeycomb
-
摘要: 采用等体积共浸渍法制备了CuO-CeO2整体式催化剂, 评价了催化剂对乙酸乙酯、异丙醇及甲苯的催化燃烧性能.采用N2吸附-脱附、X射线衍射 (XRD)、氢气程序升温还原 (H2-TPR)、氨气程序升温脱附 (NH3-TPD) 以及挥发性有机化合物脱附等手段对催化剂进行了表征.表征数据显示, 氧化铜以高分散态均匀分散存在于载体表面, 氧化铈则是小的纳米颗粒, 氧化铈颗粒粒径随着Cu/Ce物质的量比的减小而增大.添加铈氧化物会显著增加总酸量, 特别是路易斯酸酸位的量, 同时增强了乙酸乙酯和异丙醇的吸附量, 吸附量的增加提高了催化剂对乙酸乙酯和异丙醇的催化燃烧性能.从甲苯的催化燃烧实验可以看出, 大量添加CeO2稍微增加了甲苯的吸附容量, 减弱了催化剂的还原性、降低了活性氧的含量, 最终导致甲苯的低转化率.催化行为由氧化铜、氧化铈以及载体三者之间的共同作用决定, 这三者的协同作用不仅影响着表面氧的活性同时影响着催化剂对甲苯的吸附能力.Abstract: CuO-CeO2 monolithic catalysts supported on SiO2-Al2O3 modified glass-fiber honeycomb were prepared via co-impregnation method and their performance in the oxidation of volatile organic compounds (VOCs) such as ethyl acetate, isopropanol and toluene was evaluated. Various techniques such as N2 sorption, X-ray powder diffraction (XRD), hydrogen-temperature programmed reduction (H2-TPR), ammonia-temperature programmed desorption (NH3-TPD) and chemisorption of VOCs were employed to characterize the catalysts. The results show that the copper oxide species are highly dispersed on the CuO-CeO2 based catalysts; moreover, the size of CeO2 nanoparticles increases with the decrease of copper/ceria molar ratio. The addition of ceria oxide can evidently increase the amount of total acid sites, especially the Lewis ones, which can enhance the adsorption capacity of ethyl acetate and isopropanaol and promote the oxidation of ethyl acetate and isopropanaol. In the case of toluene combustion, the addition of large amount of CeO2 may decrease the reducibility and oxygen activation capability; as a result, it contributes little to the adsorption of toluene, resulting in a low activity in the oxidation of toluene. The catalytic activity is related both to the reactivity of surface oxygen and to the adsorption capacity of the catalyst towards VOC molecules, which are determined by the complex interactions among copper, cerium oxide and the support.
-
Key words:
- volatile organic compounds /
- catalytic oxidation /
- ethyl acetate /
- isopropanol /
- toluene /
- CuO /
- CeO2 /
- glass-fiber honeycomb
-
Table 1 Properties of various catalysts
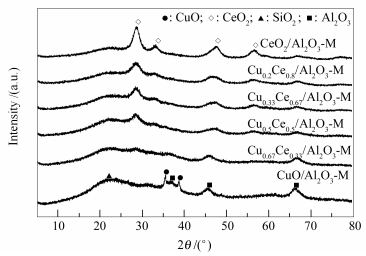
Catalyst A/ (m2·g-1) vp / (cm3·g-1) dp / nma DC/ nmb H2 consumed/ (mmol·g-1) Acidity/(μmol·g-1)c Adsorption capacity/ (μmol·g-1) Lewis Brønsted total ethyl acetate isopropanol toluene CuO/Al2O3-M 58 0.130 9.0 - 1.20 1 099 307 1 406 251 1 012 52 Cu0.67Ce0.33/Al2O3-M 56 0.125 8.9 - 0.61 1 299 384 1 683 281 1 294 56 Cu0.5Ce0.5/Al2O3-M 53 0.123 9.3 4.5 0.48 1 456 279 1 735 334 1 424 66 Cu0.33Ce0.67/Al2O3-M 50 0.117 9.4 4.8 0.40 1 352 276 1 628 279 1 153 61 Cu0.2Ce0.8/Al2O3-M 44 0.112 10.2 4.6 0.13 1 340 281 1 621 307 1 258 59 CeO2/Al2O3-M 36 0.101 11.2 5.8 0.06 1 291 273 1 561 261 1 280 50 a: pore diameter determined with the nitrogen desorption isotherms by the BJH method;
b: particle sizes estimated from the (111) ceria diffraction peaks by using Scherrer equation;
c: acidity determined by NH3-TPD -
[1] LIOTTA L F. Catalytic oxidation of volatile organic compounds on supported noble metals[J]. Appl Catal B:Environ, 2010, 100(3/4):403-412. http://www.sciencedirect.com/science/article/pii/S0926337310003760 [2] LI W H, GONG H. Recent progress in the removal of volatile organic compounds by catalytic combustion[J]. Acta Phys Chim Sin, 2010, 26(4):885-894. https://www.researchgate.net/publication/262824167_Recent_Progress_in_the_Removal_of_Volatile_Organic_Compounds_by_Catalytic_Combustion [3] SAQER S M, KONDARIDES D I, VERYKIOS X E. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on Al2O3[J]. Appl Catal B:Environ, 2011, 103(3/4):275-286. doi: 10.1016/j.apcatb.2011.01.001 [4] KIM S C, SHIM W G. Catalytic combustion of VOCs over a series of manganese oxide catalysts[J]. Appl Catal B:Environ, 2010, 98(3/4):180-185. https://www.researchgate.net/publication/232403887_Catalytic_combustion_of_VOCs_over_a_series_of_manganese_oxide_catalysts [5] LARSSON P O, ANDERSSON A, WALLENBERG L R, SVENSSONY B. Combustion of CO and toluene; Characterisation of copper oxide supported on titania and activity comparisons with supported cobalt, iron, and manganese oxide[J]. J Catal, 1996, 163(2):279-293. doi: 10.1006/jcat.1996.0329 [6] BIAŁAS A, KONDRATOWICZ T, DROZDEK M, KU'STROWSKI P. Catalytic combustion of toluene over copper oxide deposited on two types of yttria-stabilized zirconia[J]. Catal Today, 2015, 257(1):144-149. https://www.researchgate.net/publication/272365520_Catalytic_combustion_of_toluene_over_copper_oxide_deposited_on_two_types_of_yttria-stabilized_zirconia [7] KIM S C. The catalytic oxidation of aromatic hydrocarbons over supported metal oxide[J]. J Hazard Mater, 2002, 91(1/3):285-299. https://www.researchgate.net/publication/11462840_The_Catalytic_Oxidation_of_Aromatic_Hydrocarbons_Over_Supported_Metal_Oxide [8] LIU S, WU X D, WENG D, RAN R. Ceria-based catalysts for soot oxidation:A review[J]. J Rare Earth, 2015, 33(6):567-590. doi: 10.1016/S1002-0721(14)60457-9 [9] DELIMARIS D, IOANNIDES T. VOC oxidation over CuO-CeO2 catalysts prepared by a combustion method[J]. Appl Catal B:Environ, 2009, 89(1/2):295-302. https://www.researchgate.net/publication/223913749_VOC_oxidation_over_CuO-CeO2_catalysts_prepared_by_a_combustion_method [10] TSONCHEVA T, ISSA G, BLASCO T, DIMITROV M, POPOVA M, HERNÁNDEZ S, KOVACHEVA D, ATANASOVA G, LÓPEZ NIETO J M. Catalytic VOCs elimination over copper and cerium oxide modified mesoporous SBA-15 silica[J]. Appl Catal A:Gen, 2013, 453:1-12. doi: 10.1016/j.apcata.2012.12.007 [11] LOPATIN S, MIKENIN P, PISAREV D, BARANOV D, ZAZHIGALOV S, ZAGORUIKO A. Pressure drop and mass transfer in the structured cartridges with fiber-glass catalyst[J]. Chem Eng J, 2015, 282:58-65. doi: 10.1016/j.cej.2015.02.026 [12] PEI T J, LIU L S, XU L K, LI Y, HE D. A novel glass fiber catalyst for the catalytic combustion of ethyl acetate[J]. Catal Commun, 2016, 74:19-23. doi: 10.1016/j.catcom.2015.10.030 [13] LIU L S, LIU Z Y, YANG J L, HUANG Z G, LIU Z H. Effect of preparation conditions on the properties of a coal-derived activated carbon honeycomb monolith[J]. Carbon, 2007, 45(14):2836-2842. doi: 10.1016/j.carbon.2007.08.006 [14] ANDRADE-MARTÍNEZ J, ORTEGA-ZARZOSA G, GÓ MEZ-CORTÉ S A, RODRÍGUEZ-GONZÁLEZ V. N2O catalytic reduction over different porous SiO2 materials functionalized with copper[J]. Powder Technol, 2015, 274:305-312. doi: 10.1016/j.powtec.2015.01.048 [15] SEDJAME H J, FONTAINE C, LAFAYE G, BARBIER JR J. On the promoting effect of the addition of ceria to platinum based alumina catalysts for VOCs oxidation[J]. Appl Catal B:Environ, 2014, 144(1):233-242. https://www.researchgate.net/publication/256093995_On_the_promoting_effect_of_the_addition_of_ceria_to_platinum_based_catalysts_for_VOCs_oxidation [16] WAN H Q, WANG Z, ZHU J, LI X W, LIU B, GAO F, DONG L, CHEN Y. Influence of CO pretreatment on the activities of CuO/γ-Al2O3 catalysts in CO+O2 reaction[J]. Appl Catal B:Environ, 2008, 79(3):254-261. doi: 10.1016/j.apcatb.2007.10.025 [17] JIANG M H, WANG B W, YAO Y Q, LI Z H, MA X B, QIN S D, SUN Q. A comparative study of CeO2-Al2O3 support prepared with different methods and its application on MoO3/CeO2-Al2O3 catalyst for sulfur-resistant methanation[J]. Appl Surf Sci, 2013, 285:267-277. doi: 10.1016/j.apsusc.2013.08.049 [18] ZHANG S M, HUANG W P, QIU X H, LI B Q, ZHENG X C, WU S H. Comparative study on catalytic properties for low-temperature CO oxidation of Cu/CeO2 and CuO/CeO2 prepared via solvated metal atom impregnation and conventional impregnation[J]. Catal Lett, 2002, 80(1/2):41-46. doi: 10.1023/A:1015318525080 [19] BERA P, ARUNA S T, PATIL K C, HEGDE M S. Studies on Cu/CeO2:A new NO reduction catalyst[J]. J Catal, 1999, 186(1):36-44. doi: 10.1006/jcat.1999.2532 [20] HOČEVAR S, KRAŠOVEC U O, OREL B, ARICÓ A S, KIM H. CWO of phenol on two differently prepared CuO-CeO2 catalysts[J]. Appl Catal B:Environ, 2000, 28(2):113-125. doi: 10.1016/S0926-3373(00)00167-3 [21] TANG X L, ZHANG B C, LI Y, XU Y D, XIN Q, SHEN W J. Carbon monoxide oxidation over CuO/CeO2 catalysts[J]. Catal Today, 2004, 93/95:191-198. doi: 10.1016/j.cattod.2004.06.040 [22] BERA P, PRIOLKAR K R, SARODE P R, HEGDE M S, EMURA S, KUMASHIRO R, LALLA N P. Structural investigation of combustion synthesized Cu/CeO2 catalysts by EXAFS and other physical techniques:Formation of a Ce1-xCuxO2-δ solid solution[J]. Chem Mater, 2002, 14(8):3591-3601. doi: 10.1021/cm0201706 [23] JIANG X Y, LU G L, ZHOU R X, MAO J X, CHEN Y, ZHENG X M. Studies of pore structure, temperature-programmed reduction performance, and microstructure of CuO/CeO2 catalysts[J]. Appl Surf Sci, 2001, 173(3/4):208-220. [24] GIORDANO F, TROVARELLI A, DE LEITENBURG C, GIONA M. A model for the temperature-programmed reduction of low and high surface area ceria[J]. J Catal, 2000, 193(2):273-282. doi: 10.1006/jcat.2000.2900 [25] LAI S Y, QIU Y F, WANG S J. Effects of the structure of ceria on the activity of gold/ceria catalysts for the oxidation of carbon monoxide and benzene[J]. J Catal, 2006, 237(2):303-313. doi: 10.1016/j.jcat.2005.11.020 [26] HU C Q. Enhanced catalytic activity and stability of Cu0.13Ce0.87Oy catalyst for acetone combustion:Effect of calcination temperature[J]. Chem Eng J, 2010, 159(1/3):129-137. [27] ARENA F, DARIO R, PARMALIANA A. A characterization study of the surface acidity of solid catalysts by temperature programmed methods[J]. Appl Catal A:Gen, 1998, 170:127-137. doi: 10.1016/S0926-860X(98)00041-6 [28] ROY S, HEGDE M S, MADRAS G. Catalysis for NOx abatement[J]. Appl Energy, 2009, 86(11):2283-2297. doi: 10.1016/j.apenergy.2009.03.022 [29] LEE K J, KUMAR P A, MAQBOOL M S, RAO K N, SONG K H, HA H P. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR:Physico-chemical properties and catalytic activity[J]. Appl Catal B:Environ, 2013, 142/143(10):705-717. [30] CHMIELARZ L, DZIEMBAJ R, GRZYBEK T, KLINIK J, ŁOJEWSKI T, OLSZEWSKA D, WEGRZYN A. Pillared smectite modified with carbon and manganese as catalyst for SCR of NOx with NH3. Part Ⅱ. Temperature-programmed studies[J]. Catal Lett, 2000, 70(1):51-56. [31] XU H Y, CHEN Y X, LI W Z. The effect of supports on the activity of methane dissociation over Rh catalysts[J]. Chin J Catal, 2007, 28(4):293-295. doi: 10.1016/S1872-2067(07)60026-6 [32] KIWI-MINSKER L, BULUSHEV D A, RAINONE F, RENKEN A. Implication of the acid-base properties of V/Ti-oxide catalyst in toluene partial oxidation[J]. J Mol Catal A:Chem, 2002, 184(1/2):223-235. https://www.researchgate.net/publication/222707059_Implication_of_the_Acid-Base_Properties_of_VTi-oxide_Catalyst_in_Toluene_Partial_Oxidation [33] DE RIVAS B, SAMPEDRO C, LÓPEZ-FONSECA R, GUTIÉ RREZ-ORTIZ MÁ, GUTIÉ RREZ-ORTIZ J I. Low-temperature combustion of chlorinated hydrocarbons over CeO2/HZSM5 catalysts[J]. Appl Catal A:Gen, 2012, 417/418:93-101. doi: 10.1016/j.apcata.2011.12.028 [34] LIN L Y, BAI H. Promotional effects of manganese on the structure and activity of Ce-Al-Si based catalysts for low-temperature oxidation of acetone[J]. Chem Eng J, 2016, 291:94-105. doi: 10.1016/j.cej.2016.01.098 [35] CARABINEIRO S A C, CHEN X, MARTYNYUK O, BOGDANCHIKOVA N, AVALOS-BORJA M, PESTRYAKOV A, TAVARES P B, ÓRFÃ O J J M, PEREIRA M F R, FIGUEIREDO J L. Gold supported on metal oxides for volatile organic compounds total oxidation[J]. Catal Today, 2015, 244:103-114. doi: 10.1016/j.cattod.2014.06.034 [36] LIANG C J, FANG J W. Predicting the kinetics of catalytic oxidation of multicomponent organic waste gases[J]. Chem Eng Sci, 2016, 144:101-107. doi: 10.1016/j.ces.2016.01.038 -





 下载:
下载: