-
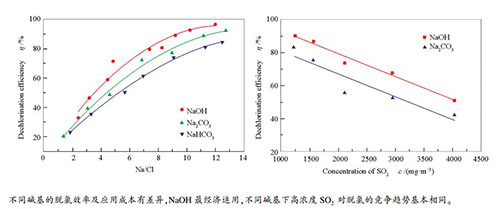
摘要: 实验探究NaOH、Na2CO3、NaHCO3这三种常见的碱基物质在模拟燃煤烟气中的实际表现,发现三种碱基物质均具有一定的脱氯性能,NaOH、Na2CO3、NaHCO3的脱氯性能依次下降,以脱氯效率70%为目标,使用三种碱基物质Na/Cl比分别需要达到5.8、7.1、8.7。高浓度SO2的存在对烟气脱氯有竞争作用,随着SO2浓度的提高,脱氯效率线性下降,不同碱基物质下,SO2浓度对脱氯效率的影响规律基本一致,SO2浓度每增加100 mg/m3,脱氯效率下降约1.4%。由于三种碱基物质达到相同脱氯效率时的Na/Cl比不同,综合考虑成本和溶解性,NaOH最具工业应用价值。Abstract: Three common alkali-based materials, NaOH, Na2CO3 and NaHCO3, were utilized to explore their dechlorination performance in a simulated coal-fired flue gas. The results show that the dechlorination efficiency increases along with the enhancement of alkaline intensity. As the Na/Cl molar ratio reaches 5.8, 7.1 and 8.7, respectively, the dechlorination efficiency of all the three alkalis (NaOH, Na2CO3 or NaHCO3) exceeds 70%. The SO2 of high concentration in flue gas has competitive effects on dechlorination. With the increase in SO2 concentration, the dechlorination efficiency drops linearly. The influence of SO2 concentration on the dechlorination efficiency is almost identical regardless of different alkali-based materials. For per 100 mg/m3 augment in SO2 concentration, the dechlorination efficiency decreases by about 1.4%. NaOH is determined to be the most valuable alkali-based material for industrial application considering the cost and solubility.
-
Key words:
- coal-fired flue gas /
- dechlorination /
- zero discharge of desulfurization wastewater /
- NaOH /
- Na2CO3 /
- NaHCO3
-
表 1 烟气中酸性气体的性质
Table 1 Properties of acid gases in flue gas
SO2 SO3 HCl HF CO2 Activity medium strong strong weak weak Concentration high low low low high Dissolubility low high high high high 表 2 燃煤机组空预器后烟气组分
Table 2 Composition of flue gas
Composition CO2 φ/% O2 φ/% SO2/(mg·m-3) SO3/(μL·L-1) HCl/(μL·L-1) HF/(μL·L-1) Concentration 14.0 3.0 2285.7 9.0 33.5 28.4 φ: volume fraction 表 3 不同碱基物质的成本
Table 3 Cost of different alkali-based materials
Alkali-based
materialsMarket
average
price
(yuan/ton)Na/Cl
at 70%
dechlorination
efficiencyPrice of
removing
1 mol
HCl(yuan)NaOH 4500 5.8 1.04 Na2CO3 2400 7.1 1.69 NaHCO3 1700 8.7 3.29 表 4 不同碱基物质的溶解性
Table 4 Dissolubility of different alkali-based materials
Temperature t/℃ 0 10 20 30 40 NaOH dissolubility /(g·100 mL-1) 42 51 109 119 129 saturation concentration/% 29.6 33.8 52.2 54.3 56.3 Na2CO3 dissolubility /(g·100 mL-1) 7 12.5 21.5 39.7 49 saturation concentration/% 6.5 11.1 17.7 28.4 32.9 NaHCO3 dissolubility /(g·100 mL-1) 6.9 8.2 9.6 11.1 12.7 saturation concentration/% 6.5 7.6 8.8 10.0 11.3 -
[1] 李玉, 张乔, 王群.蒸发结晶工艺在火电厂脱硫废水零排放中的应用[J].水处理技术, 2016, 425(11):121-122. http://mall.cnki.net/magazine/Article/XBDJ201408022.htmLI Yu, ZHANG Qiao, WANG Qun. Application of evaporative crystallization process in the zero discharge of desulfurization waste water in thermal power plant[J]. Technol Water Treat, 2016, 42(11):121-122. http://mall.cnki.net/magazine/Article/XBDJ201408022.htm [2] CINGOLANI D, EUSEBI A L, BATTISTONI P. Osmosis process for leachate treatment in industrial platform:Economic and performances evaluations to zero liquid discharge[J]. J Environ Manage, 2017, 203:782-790. doi: 10.1016/j.jenvman.2016.05.012 [3] MA S, CHAI J, CHEN G, YU W, ZHU S. Research on desulfurization waste-water evaporation:Present and future perspectives[J]. Renewable Sustainable Energy Rev, 2016, 58:1143-1151. doi: 10.1016/j.rser.2015.12.252 [4] 吴怡卫.石灰石-石膏湿法烟气脱硫废水处理的研究[J].中国电力, 2006, 39(4):75-78. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdl200604018WU Yi-wei. Study on the wastewater treatment in limestone-gypsum wet FGD process[J]. Electr Pow, 2006, 39(4):75-78. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdl200604018 [5] 杨建国, 耿梓文, 袁伟中, 陈锡炯, 滕卫明, 刘畅, 赵虹.燃煤烟气脱氯实现脱硫废水零排放技术及其影响[J].中国电机工程学报, 2018, 38(9):2657-2664. https://www.cnki.com.cn/qikan-MTZH201703011.htmlYANG Jian-guo, GENG Zi-wen, YUAN Wei-zhong, CHEN Xi-jiong, TENG Wei-ming, LIU Chang, ZHAO Hong. The technology of coal-fired flue gas dechlorination for realizing zero-discharge of desulfurization wastewater and its influences on boiler[J]. Proc CSEE, 2018, 38(9):2657-2664. https://www.cnki.com.cn/qikan-MTZH201703011.html [6] LIU C, ZHAO H, YANG W Y, QIU K Z, YANG J G, GENG Z W, TENG W M, YUAN W Z, CHEN X J. Chemical kinetics simulation of semi-dry dechlorination in coal-fired flue gas[J]. J Zhejiang Univ-Sci A, 2018, 2(19):148-157. [7] 解海卫, 张于峰, 张艳.垃圾焚烧电厂烟气脱酸数值模拟及实验研究[J].中国电机工程学报, 2008, 28(5):17-22. https://cnki.com.cn/qikan-ZGDC200805004.htmlJIE Hai-wei, ZHANG Yu-feng, ZHANG Yan. Numerical simulation and experimental study of flue gas cleaning in waste incineration power plants[J]. Proc CSEE, 2008, 28(5):17-22. https://cnki.com.cn/qikan-ZGDC200805004.html [8] 臧仁德, 张力.垃圾与煤混烧烟气脱酸的模拟及实验[J].煤炭学报, 2011, 36(8):1385-1390. http://www.oalib.com/paper/4234290ZANG Ren-de, ZHANG Li. Numerical simulation and experimental on deacidification of flue gas by co-combustion of MSW with coal[J]. J China Coal Soc, 2011, 36(8):1385-1390. http://www.oalib.com/paper/4234290 [9] ZHANG C X, WANG Y X, YANG Z H, XU M H. Chlorine emission and dechlorination in co-firing coal and the residue from hydrochloric acid hydrolysis of discorea zingiberensis[J]. Fuel, 2006, 85(14/15):2034-2040. [10] FRIGGE L, STROEHLE J, EPPLE B. Release of sulfur and chlorine gas species during coal combustion and pyrolysis in an entrained flow reactor[J]. Fuel, 2017, 201:105-110. doi: 10.1016/j.fuel.2016.11.037 [11] LI W, LU H L, CHEN H K, LI B Q. The volatilization behavior of chlorine in coal during its pyrolysis and CO2-gasification in a fluidized bed reactor[J]. Fuel, 2005, 84(14/15):1874-1878. [12] TSUBOUCHI N, OHTSUKA S, NAKAZATO Y, OHTSUKA Y. Formation of hydrogen chloride during temperature-programmed pyrolysis of coals with different ranks[J]. Energy Fuels, 2005, 19(2):554-560. doi: 10.1021/ef040077z [13] GUO S Q, YANG J L, LIU Z Y. The fate of fluorine and chlorine during thermal treatment of coals[J]. Environ Sci Technol, 2006, 40(24):7886-7889. doi: 10.1021/es0604562 [14] BIE R S, LI S Y, YANG L D. Reaction mechanism of CaO with HCl in incineration of wastewater in fluidized bed[J]. Chem Eng Sci, 2005, 60(3):609-616. doi: 10.1016/j.ces.2004.08.022 [15] SUN Z C, YU F C, LI F X, LI S G, FAN L S. Experimental study of HCl capture using CaO sorbents:Activation, deactivation, reactivation, and ionic transfer mechanism[J]. Ind Eng Chem Res, 2011, 50(10):6034-6043. doi: 10.1021/ie102587s [16] VERDONE N, DE FILIPPIS P. Thermodynamic behaviour of sodium and calcium based sorbents in the emission control of waste incinerators[J]. Chemosphere, 2004, 54(7):975-985. doi: 10.1016/j.chemosphere.2003.09.041 [17] FELLOWS K T, PILAT M J. HCl sorption by dry NaHCO3 for incinerator emissions control[J]. J Air Waste Manage, 1990, 40(6):887-893. doi: 10.1080/10473289.1990.10466734 [18] 李猛. 钠法烟气脱硫脱硝一体化技术[C]//煤电厂"超低排放"新技术交流研讨会论文. 中国浙江嘉兴: 中国动力工程学会, 2014: 38-45.LI Meng. A integrated technology of flue gas desulfurization and denitrification by sodium based materials[C]//Processings of new technology exchange seminar of ultra-low emissions in coal-fired plant. Jiaxing, Zhejiang, China: Chinese Society of Power Engineering, 2014: 38-45. [19] 王永刚, 李振虎, 张文胜, 曾东, 郭锴.用旋转填充床以双碱法脱除烟气中的SO2[J].石油化工, 2009, 38(8):893-896.WANG Yong-gang, LI Zhen-hu, ZHANG Wen-sheng, CENG Dong, GUO Kai. Removal of sulfur dioxide from flue gas in rotating packed bed by dual-alkali method[J]. Petrochem Technol, 2009, 38(8):893-896. [20] LEWIS W K, WHITMAN W G. Principles of gas absorption[J]. Ind Eng Chem, 1924, 16:1215-1220. doi: 10.1021/ie50180a002 [21] LIU Z S, WEY M Y, LIN C L. Reaction characteristics of Ca(OH)2, HCl and SO2 at low temperature in a spray dryer integrated with a fabric filter[J]. J Hazard Mater, 2002, 95(3):291-304. doi: 10.1016/S0304-3894(02)00142-5 [22] STEIN J, KIND M, SCHLUNDER E U. The influence of HCl on SO2 absorption in the spray dry scrubbing process[J]. Chem Eng J, 2002, 86(1/2):17-23. doi: 10.1631/jzus.A1600653 [23] HARTMAN M, SVOBODA K, POHORELY M, SYC M, SKOBLIA S, CHEN P C. Reaction of hydrogen chloride gas with sodium carbonate and its deep removal in a fixed-bed reactor[J]. Ind Eng Chem Res, 2014, 53(49):19145-19158. doi: 10.1021/ie503480k [24] 刘畅, 赵虹, 滕卫明, 耿梓文, 邱坤赞, 杨建国, 袁伟中, 陈锡炯. n(Na+)/n(Cl-)对烟气脱氯及脱硫废水零排放的影响[J].煤炭转化, 2017, 40(3):70-75.LIU Chang, ZHAO Hong, TENG Wei-ming, GENG Zi-wen, QIU Kun-zan, YANG Jian-guo, YUAN Wei-zhong, CHEN Xi-jiong. Effect of n(Na+)/n(C1-) on semi-dry dechlorination in coal-fired flue gas to realize zero emission of FGD waste water[J]. Coal Convers, 2017, 40(3):70-75. -





 下载:
下载: