Experimental study on co-gasification reactivity of Shenfu bituminous coal char and MSW-based hydrochar
-
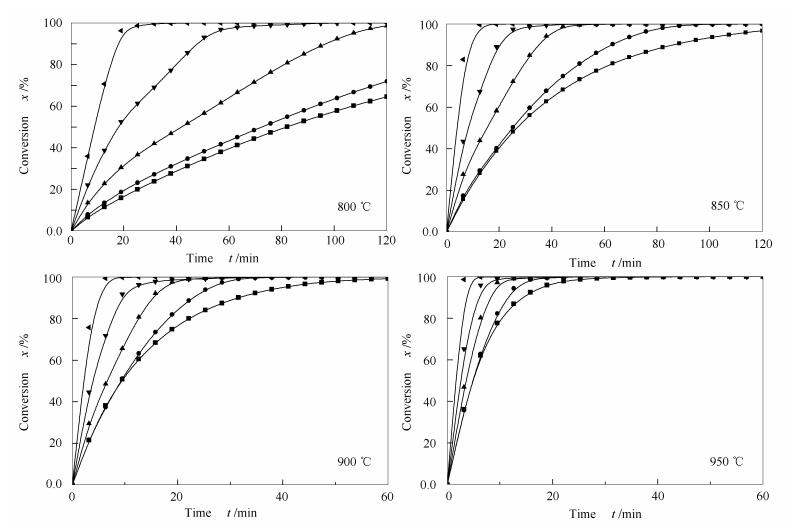
摘要: 基于常压热重分析仪(TG)开展了神府烟煤焦(SF char)、水热炭焦(HTC char)及其混合物等温CO2气化实验以研究气化温度(800-950℃)、掺混比(3:1、1:1、1:3)对共气化特性的影响,并探讨了气化反应活化能及其影响因素。结果表明,HTC因其较大的比表面积和较多的灰分而具有较强的气化活性。低HTC掺混比的混合物气化活性对温度变化敏感。低温下混合物的气化活性受HTC掺混影响显著。反应活化能随着反应转化率的增大而逐渐增大并趋于稳定。进一步研究表明,混合物的活化能与其掺混比以及活性矿物(K+Na)/Ca的物质的量比均存在近似线性关系。Abstract: The influences of gasification temperature (800-950℃) and blending ratio (3:1, 1:1, 1:3) on the isothermal CO2 co-gasification reactivities of Shenfu bituminous coal char (SF char) and HTC char were investigated using an atmospheric thermogravimetric analyzer (TGA). Moreover, the activation energy of char gasification and its influence factors were explored. The results show that the greater surface area and the higher ash content are the main reasons for the high gasification reactivity of HTC char. The reactivities of mixtures with low HTC char proportion are more sensitive to temperature at low temperature range. The activation energy increases with the increase of carbon conversion, and the activation energy correlates well with the blending ratio and the molar ratio of active (K+Na)/Ca in the char.
-
Key words:
- co-gasification /
- coal /
- hydrochar /
- reactivity /
- activation energy
-
表 1 样品的工业分析和元素分析
Table 1 Proximate analysis, ultimate analysis and ash fusion temperature of the samples tested
Sample Proximate analysis wd/% Ultimate analysis wd/% V FC A C H N O* St SF 35.42 58.29 6.29 79.14 2.32 1.12 10.36 0.77 SF char 6.48 82.255 10.27 87.17 0.91 0.54 1.01 0.10 HTC 69.71 11.38 18.91 47.36 8.56 1.78 23.01 0.38 HTC char 8.65 54.99 36.36 56.71 0.77 1.03 4.98 0.15 d:dry basis;*:by difference 表 2 样品的灰成分分析
Table 2 Ash composition of samples
Sample Ash composition w/% SiO2 Al2O3 K2O Na2O CaO Fe2O3 MgO SF 33.36 12.44 0.67 1.73 27.78 9.11 1.34 HTC 20.22 8.07 3.58 4.40 37.43 5.14 2.06 表 3 热解前后样品质量及混合比例变化
Table 3 Quality and blending ratio change of samples during pyrolysis
SF m/g HTC m/g Mixing ratio (SF:HTC) Raw material 20.00 20.00 3:1 1:1 1:3 Char 13.44 7.17 0.85:0.15 0.65:0.35 0.38:0.62 表 4 SF半焦表面的元素组成
Table 4 Element composition on the surface of AF semi-char
Content w /% Na Mg Al Si K Ca Fe Co-gasificationa, b 5.66 0.99 2.08 3.70 2.64 2.36 0.83 Individual gasification 1.33 0.49 1.62 2.24 0.32 1.84 0.83 a:SF semi-char was obtained from the mixture SF:HTC-1:1-800P
b:At the same gasification time, the conversions were xSF:HTC-1:1-800P=0.9 and xSF-800P=0.67 respectively表 5 不同转化率活化能拟合的校正决定系数
Table 5 The Adj. R. Square (R2) of activation energy under different conversion
Sample R2 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 SF-800P 0.978 0.983 0.986 0.989 0.993 0.995 0.997 0.998 0.999 SF:HTC-3:1-800P 0.979 0.987 0.991 0.993 0.994 0.994 0.995 0.995 0.996 SF:HTC-1:1-800P 0.970 0.986 0.992 0.995 0.995 0.996 0.996 0.996 0.996 SF:HTC-1:3-800P 0.960 0.981 0.988 0.991 0.993 0.995 0.994 0.994 0.994 HTC-800P 0.986 0.982 0.985 0.987 0.988 0.990 0.991 0.990 0.989 表 6 气化反应的活化能
Table 6 Gasification activation energy
Conversion Gasification activation energy EA/(kJ·mol-1) SF-800P SF-HTC-3:1-800P SF-HTC-1:1-800P SF-HTC-1:3-800P HTC-800P 0.1 192.34 176.16 149.62 133.11 114.43 0.2 201.85 187.42 163.05 142.04 126.83 0.3 207.31 195.00 169.71 147.46 133.91 0.4 210.08 199.28 177.10 150.39 137.06 0.5 211.30 201.22 182.80 152.48 139.89 0.6 211.73 201.95 185.28 158.17 141.48 0.7 211.85 202.31 186.04 163.59 140.86 0.8 211.63 203.10 186.07 164.66 139.94 0.9 211.15 204.30 185.35 162.75 139.80 Average value 207.69 196.75 176.11 152.74 134.91 表 7 焦活性AAEM含量
Table 7 Content of active AAEM in char
AAEM w/(mmol·g-1) SF-800P SF-HTC-3:1-800P* SF-HTC-1:1-800P* SF-HTC-1:3-800P* HTC-800P K 0.0015 0.0472 0.1082 0.1904 0.3062 Na 0.0061 0.0441 0.0948 0.1632 0.2596 Ca 0.5000 0.5705 0.6646 0.7916 0.9703 -
[1] 李位位, 黄戒介, 王志青, 段会文, 李俊国, 房倚天.煤焦CO2气化反应动力学及内扩散对气化过程的影响分析[J].燃料化学学报, 2016, 44(12):1416-1421.LI Wei-wei, HUANG Jie-jie, WANG Zhi-qing, DUANG Hui-wen, LI Jun-guo, FANG Yi-tian. Reaction kinetics of coal char gasification with CO2 and the effect of internal diffusion on the gasification[J]. J Fuel Chem Technol, 2016, 44(12):1416-1421. [2] MOON J, MUN T-Y, YANG W, LEE U, HWANG J, JANG E, CHOI C. Effects of hydrothermal treatment of sewage sludge on pyrolysis and steam gasification[J]. Energy Convers Manage, 2015, 89:401-407. http://www.sciencedirect.com/science/article/pii/S0196890415005993 [3] MASNADI M S, GRACE J R, BI X T, JIM L, NAOKO E. From fossil fuels towards renewables:Inhibitory and catalytic effects on carbon thermochemical conversion during co-gasification of biomass with fossil fuels[J]. Appl Energy, 2015, 140(15):196-209. https://www.cabdirect.org/cabdirect/abstract/20153285884 [4] JEONG H J, PARK S S, HWANG J. Co-gasification of coal-biomass blended char with CO2 at temperatures of 900-1100℃[J]. Fuel, 2014, 116(15):465-470. http://www.wenkuxiazai.com/doc/14ef2cc4aaea998fcd220e1c.html [5] SATYAM N V, AGHALAYAM P, JAYANTI S. Synergetic and inhibition effects in carbon dioxide gasification of blends of coals and biomass fuels of Indian origin[J]. Bioresour Technol, 2016, 209:157-165. doi: 10.1016/j.biortech.2016.02.137 [6] ZHANG Z, PANG S, LEVI T. Influence of AAEM species in coal and biomass on steam co-gasification of chars of blended coal and biomass[J]. Renew Energy, 2017, 101:356-363. doi: 10.1016/j.renene.2016.08.070 [7] RIZKIANA J, GUAN G, WIDAYATNO W B, HAO X G, LI X M, HUANG W, ABULITY A. Promoting effect of various biomass ashes on the steam gasification of low-rank coal[J]. Appl Energy, 2014, 133(15):282-288. http://www.sciencedirect.com/science/article/pii/S030626191400782X [8] WEI J T, GUO Q H, HE Q, DING L, Yoshikawa K, YU G S. Co-gasification of bituminous coal and hydrochar derived from municipal solid waste:Reactivity and synergy[J]. Bioresour Technol, 2017, 239:482-489. doi: 10.1016/j.biortech.2017.05.014 [9] HUO W, ZHOU Z J, WANG F C, WANG Y F, YU G S. Experimental study of pore diffusion effect on char gasification with CO2 and steam[J]. Fuel, 2014, 131(1):59-65. http://www.sciencedirect.com/science/article/pii/S0016236114003937 [10] WEI X F, HUANG J J, LIU T F, FANG Y T, WANG Y. Transformation of alkali metals during pyrolysis and gasification of a lignite[J]. Energy Fuel, 2008, 22(3):1840-1844. doi: 10.1021/ef7007858 [11] WEI J T, GUO Q H, CHEN H D, CHEN X L, YU G S. Study on reactivity characteristics and synergy behaviours of rice straw and bituminous coal co-gasification[J]. Bioresour Technol, 2016, 220:509-515. doi: 10.1016/j.biortech.2016.08.116 [12] 陈凡敏, 王兴军, 王西明, 周志杰.煤催化气化过程中钾的迁移及其对气化反应特性的影响[J].燃料化学学报, 2013, 41(3):265-270. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18132.shtmlCHEN Fan-min, WANG Xing-jun, WANG Xi-ming, ZHOU Zhi-jie. Transformation of potassium during catalytic gasification of coal and the effect on gasification[J]. J Fuel Chem Technol, 2013, 41(3):265-270. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18132.shtml [13] DING L, ZHANG Y Q, WANG Z Q, HUANG J J, FANG Y T. Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char[J]. Bioresour Technol, 2014, 173:11-20. doi: 10.1016/j.biortech.2014.09.007 [14] MAHINPEY N, GOMEZ A. Review of gasification fundamentals and new findings:Reactors, feedstock, and kinetic studies[J]. Chem Eng Sci, 2016, 148(12):14-31. http://www.sciencedirect.com/science/article/pii/S0009250916301440 [15] GOMEZ A, MAHINPEY N. A new method to calculate kinetic parameters independent of the kinetic model:Insights on CO2 and steam gasification[J]. Chem Eng Res Des, 2015, 95:346-357. doi: 10.1016/j.cherd.2014.11.012 [16] 董存珍, 汪小憨, 曾小军, 邵振华. CO2气氛下生物焦气化反应动力学参数的实验研究:I.活化能[J].燃料化学学报, 2014, 42(3):329-335. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18375.shtmlDONG Cun-zhen, WANG Xiao-han, ZENG Xiao-jun, SHAO Zhen-hua. Experimental study on the gasification kinetic parameters of biomass chars under CO2 atmosphere:I. Activation energy[J]. J Fuel Chem Technol, 2014, 42(3):329-335 http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18375.shtml [17] TANNER J, BHATTACHARYA S. Kinetics of CO2 and steam gasification of Victorian brown coal chars[J]. Chem Eng J, 2016, 285(1):331-340. -





 下载:
下载: