Preparation of Pt/CeL reforming catalyst and its performance in the aromatization of naphtha
-
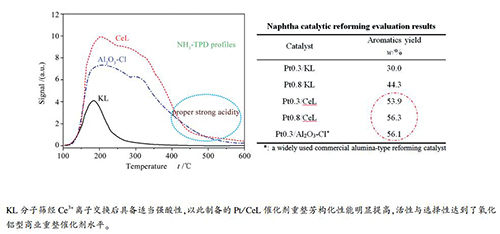
摘要: 为克服常规氧化铝重整催化剂氯离子流失及其对设备的腐蚀等问题,通过离子交换法制备了Ce3+改性的L分子筛,采用浸渍法制备了Pt/CeL重整催化剂;用XRD、N2吸附-脱附、NH3-TPD和Py-FTIR等手段对载体和催化剂进行了表征,并以硫含量为0.50 μg/mL的工业精制石脑油为原料,在固定床微型反应装置上评价了Pt/CeL催化剂的重整芳构化性能。结果表明,Ce3+离子交换可提高载体的酸量和酸强度,而不会破坏L分子筛的骨架结构;Ce3+改性后的Pt/CeL催化剂其重整芳构化性能明显提高,活性与选择性达到氧化铝型商业重整催化剂的水平,说明适当的酸性对重整催化剂芳构化反应有显著的促进作用。Abstract: To solve the problems of chlorine loss and equipment corrosion caused by the conventional alumina-type reforming catalyst, zeolite L was modified with Ce3+ by ion exchange method and with Ce3+-modified L (CeL) as the support, the Pt/CeL reforming catalyst without any chlorine was prepared by impregnation method. The CeL support and Pt/CeL catalyst were characterized by XRD, N2 adsorption-desorption, NH3-TPD and Py-FTIR; the catalytic performance of Pt/CeL in aromatization was investigated in a continuous-flow fixed-bed micro-reactor, with an industrial hydrofining naphtha containing 0.50 μg/mL sulfur as the feedstock. The results indicate that the modification with Ce3+ by ion exchange has little influence on framework structure of zeolite L; however, it can raise the acid content and acid strength of the CeL support and then remarkably enhance the performance of the Pt/CeL reforming catalyst in aromatization. The activity and selectivity of the Pt/CeL catalyst are comparable to those of the commercial alumina-type reforming catalysts, suggesting that proper acidity can facilitate the aromatization reaction.
-
Key words:
- catalytic reforming /
- cerium /
- zeolite L /
- ion exchange /
- aromatization
1) 本文的英文电子版由Elsevier出版社在ScienceDirect上出版(http://www.sciencedirect.com/science/journal/18725813). -
表 1 KL和CeL分子筛的比表面积和孔体积
Table 1 Textural properties of the KL and CeL zeolites
Sample Surface area A/(m2·g-1) Pore volume v/(mL·g-1) total micropore mesopore total micropore mesopore KL 266 220 46 0.26 0.12 0.14 CeL 266 220 46 0.24 0.11 0.13 表 2 KL、CeL和Al2O3-Cl的酸量分布
Table 2 Acid distribution of zeolite KL, zeolite CeL and Al2O3-Cl
Sample Acid amount determined by NH3-TPD/(a.u.) weak
(100-200 ℃)medium
(200-400 ℃)strong
(400-600 ℃)total
(100-600 ℃)KL 199.5 130.5 9.6 339.6 CeL 414.6 1585.4 219.6 2219.6 Al2O3-Cl 333.3 1150.8 203.9 1688.0 表 3 KL、CeL和Al2O3-Cl的B酸与L酸分布
Table 3 Lewis (L) and Brönsted (B) acid distribution of KL, CeL and Al2O3-Cl
Sample Total acid amount measured by Py-FTIR
at 200 ℃/(μmol·g-1)Strong acid amount measured by Py-FTIR
at 350 ℃/(μmol·g-1)L B B+L B/L L B B+L B/L KL 93.2 0 93.2 0 8.9 0 8.9 0 CeL 466.0 130.5 596.5 0.28 200.2 14.0 214.2 0.07 Al2O3-Cl 340.3 0 340.3 0 196.5 0 196.5 0 表 4 重整评价原料油性质
Table 4 Properties of the hydrofining naphtha feedstock
Item Result Density(20 ℃)/(g·cm-3) 0.7374 Sulfur content /(μg·mL-1) 0.50 Nitrogen content /(μg·mL-1) 0.32 PONA w/% n-paraffin 10.9 i-paraffin 43.5 Olefin 0.0 Naphthene 41.4 Aromatic 4.2 RON 76.2 表 5 Pt/KL和Pt/CeL催化剂石脑油催化重整评价
Table 5 Catalytic evaluation results of Pt/KL and Pt/CeL for naphtha reforming
Catalyst Aromatics content
in liquid products
w/%Liquid yield
w/%Total aromatics
yield w/%Benzene
yield w/%Toluene
yield w/%C8 aromatics
yield w/%C9+ aromatics
yield w/%Pt 0.3/KL 34.5 86.8 30.0 1.1 7.7 10.7 10.5 Pt 0.8/KL 53.1 83.5 44.3 1.9 12.8 15.8 13.8 Pt 0.3/CeL 65.4 82.4 53.9 2.7 15.7 19.3 16.2 Pt 0.8/CeL 70.5 79.9 56.3 3.8 17.9 19.9 14.7 Pt 0.3/Al2O3-Cl* 71.0 79.0 56.1 2.4 15.7 19.5 18.5 *:a widely used commercial alumina-type reforming catalyst;
reaction conditions: 490 ℃, 0.7 MPa(G), LHSV = 2 h-1, H2/hydrocarbon mole ratio = 9 -
[1] ANTOS G J, AITANI A M. Catalytic Naphtha Reforming:Second Edition, Revised and Expanded[M]. New York:Marcel Dekker Inc. 2004:335-349. [2] 徐承恩.催化重整工艺与工程[M].北京:中国石化出版社, 2006:1-26.XU Cheng-en. Catalytic Reforming Process and Engineering[M]. Beijing:China Petrochemical Press, 2006:1-26. [3] 马爱增.中国催化重整技术进展[J].中国科学:化学, 2014, 44(1):25-39. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QKV20152016030800040097MA Ai-zeng. Development and commercial application of naphtha catalytic reforming technology in China[J]. Sci Sin:Chim, 2014, 44(1):25-39. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QKV20152016030800040097 [4] 郭春垒, 方向晨, 贾立明, 刘全杰, 张喜文, 赵晓东.分子筛重整催化剂研究进展[J].化工进展, 2012, 31(4):825-832. http://d.old.wanfangdata.com.cn/Periodical/hgjz201204024GUO Chun-lei, FANG Xiang-chen, JIA Li-ming, LIU Quan-jie, ZHANG Xi-wen, ZHAO Xiao-dong. Development of zeolitic reforming catalyst[J]. Chem Ind Eng Prog, 2012, 31(4):825-832. http://d.old.wanfangdata.com.cn/Periodical/hgjz201204024 [5] ARENA F, FRUSTERI F, MONDELLO N, GIORDANO N, PARMALIANA A. Interaction pathway of chloride ions with γ-Al2O3:Surface acidity and thermal stability of the Cl/γ-Al2O3 system[J]. J Chem Soc, Faraday Trans, 1992, 88(22):3353-3356. doi: 10.1039/FT9928803353 [6] BESOUKHANOVA C, BARTHOMEUF D, BREYSSE M, BERNARD J R. Unusual properties of platinum alkaline zeolites in n-hexane dehydrocyclisation and benzene hydrogenation[J]. Stud Surf Sci Catal, 1981, 7:1410-1411. doi: 10.1016/S0167-2991(08)64749-7 [7] LANE G S, MODICA F S, MILLER J T. Platinum/zeolite catalyst for reforming n-hexane:kinetic and mechanistic considerations[J]. J Catal, 1991, 129(1):145-158. doi: 10.1016/0021-9517(91)90018-Y [8] JACOBS G, ALVAREZ W E, RESASCO D E. Study of preparation parameters of powder and pelletized Pt/KL catalysts for n-hexane aromatization[J]. Appl Catal A:Gen, 2001, 206(2):267-282. doi: 10.1016/S0926-860X(00)00606-2 [9] KUMAR M, SAXENA A K, NEGI B S, VISWANADHAM N. Role of pore size analysis in development of zeolite reforming catalyst[J]. Catal Today, 2008, 130(2):501-508. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1b10a4321e441d6fa86c329648a5abe0 [10] 姜贤, 郜志农, 阮振奎, 黄家生, 徐奕德, 郭燮贤, 罗锡辉, 何金海.碱性在Pt/L分子筛催化剂中的作用[J].燃料化学学报, 1994, 22(1):9-15. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199400381771JIANG Xian, GAO Zhi-nong, RUAN Zhen-kui, HUANG Jia-sheng, XU Yi-de, GUO Xie-xian, LUO Xi-hui, HE Jin-hai. The function of basicity on Pt/L zeolite reforming catalyst[J]. J Fuel Chem Technol, 1994, 22(1):9-15. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199400381771 [11] 张玉红, 薛炼, 马爱增. Pt/KL芳构化催化剂中载体酸碱性的作用[J].石油炼制与化工, 2009, 40(3):1-5. doi: 10.3969/j.issn.1005-2399.2009.03.001ZHANG Yu-hong, XUE Lian, MA Ai-zeng. Role of acidity and basicity of zeolite support in Pt/KL catalysts for aromatization of alkane[J]. Pet Process Petrochem, 2009, 40(3):1-5. doi: 10.3969/j.issn.1005-2399.2009.03.001 [12] HUGHES T R, BUSS W C, TAMM P W, JACOBSON R L. Aromatization of hydrocarbons over platinum alkaline earth zeolites[J]. Stud Surf Sci Catal, 1986, 28:725-732. doi: 10.1016/S0167-2991(09)60940-X [13] HONG S B, MIELCZARSKI E, DAVIS M E. Aromatization of n-hexane by platinum-containing molecular sieves. Part 1. Catalyst preparation by the vapor phase impregnation method[J]. J Catal, 1992, 134:349-358. doi: 10.1016/0021-9517(92)90234-9 [14] MIELCZARSKI E, HONG S B, DAVIS R J, DAVIS M E. Aromatization of n-hexane by platinum-containing molecular sieves Ⅱ. n-Hexane reactivity[J]. J Catal, 1992, 134:359-369. doi: 10.1016/0021-9517(92)90235-A [15] ARCOYA A, SEOANE X L, GRAU J M. Dehydrocyclization of n-heptane over a PtBa/KL catalyst:Reaction mechanism[J]. Appl Catal A:Gen, 2005, 284(1):85-95. http://www.sciencedirect.com/science/article/pii/S0926860X0500030X [16] 董家禄, 金长太, 须沁华.新型重整催化剂的研究-Pt/BaKL型沸石中钡的作用[J].燃料化学学报, 1992, 20(3):244-251. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000004496617DONG Jia-lu, JIN Chang-tai, XU Qin-hua. Study of new reforming catalyst-the function of Ba2+ in Pt/BaKL zeolite[J]. J Fuel Chem Technol, 1992, 20(3):244-251. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000004496617 [17] 石映祯, 姜贤, 张勇, 阮振奎, 张阳, 郭燮贤.铂/L分子筛重整催化剂烷烃芳构化反应机理[J].催化学报, 1993, 14(4):312-316. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000000017390SHI Ying-zhen, JIANG Xian, ZHANG Yong, RUAN Zhen-kui, ZHANG Yang, GUO Xie-xian. Mechanism of aromatization of alkanes over Pt/L zeolite catalysts[J]. Chin J Catal, 1993, 14(4):312-316. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000000017390 [18] MINTOVA S, BARRIER N. Verified Syntheses of Zeolitic Materials, Third Revised Edition[M]. Published on behalf of Synthesis Commission of the International Zeolite Association (ISBN:978-0-692-68539-6), 2016:271-273. [19] 霍全. L沸石中试放大合成及在烃类催化裂化催化剂中的应用研究[D].太原: 太原理工大学, 2007.HUO Quan. The pilot studies on the synthesis of zeolite L and its application in FCC catalyst[D]. Taiyuan: Taiyuan University of Technology, 2007. [20] TREACY M M J, HIGGINS J B. Collection of Simulated XRD Powder Patterns for Zeolites, Fourth Revised Edition[M]. Netherlands:Elsevier, 2001:220-221. [21] 中国科学院大连化学物理研究所分子筛组.沸石分子筛[M].北京:科学出版社, 1978:298-313.Molecular sieve section of Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Zeolite[M]. Beijing:Science Press, 1978:298-313. -





 下载:
下载: