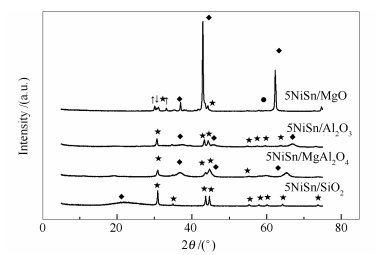
-
摘要: 研究了载体对负载型NiSn催化剂丙烷脱氢性能的影响,主要对比考察了以SiO2、MgO、Al2O3、MgAl2O4为载体的NiSn催化剂的丙烷脱氢性能。采用X射线衍射技术(XRD)、氮气吸附-脱附技术、氨气程序升温脱附技术(NH3-TPD)以及氢气程序升温还原技术(H2-TPR)对催化剂样品进行表征。结果表明,SiO2因具有较大的比表面积、大孔径、酸性较弱等特点,以其为载体制备所得催化剂中Ni2.67Sn2组分含量高,催化剂性能较高。Abstract: In this paper, we studied the effects of supports, such as SiO2, MgO, Al2O3 and MgAl2O4, on the performance of supported NiSn catalyst for propane dehydrogenation. NH3 temperature-programmed desorption, H2 temperature-programmed reduction were applied for the characterization of the catalysts. The results show that SiO2 with large specific surface area and large pore size can achieve good contact between catalyst and reactants, leading to a high content of Ni2.67Sn2 alloy and a reduced diffusion resistance, In addition, their weak acid improve dehydrogenation activity and propene selectivity.
-
Key words:
- propane dehydrogenation /
- NiSn-based catalysts /
- support
-
表 1 不同载体NiSn催化剂的结构性质
Table 1 Textural properties of NiSn catalysts with different supports
Sample ABET/(m2·g-1) vpore/(mL·g-1) dpore/nm dc/nm 5NiSn/SiO2 253 0.98 11.4 48.8 5NiSn/Al2O3 138 0.21 3.6 46.2 5NiSn/MgAl2O4 91 0.21 6.2 38.0 5NiSn/MgO 25 0.19 25.2 44.4 ABET: BET specific area; vpore: pore volume; dpore: average pore diameter; dc: crystallite size determined by scherrer’s equation 表 2 不同载体NiSn催化剂的酸量分布
Table 2 The amount of acid on NiSn catalysts with different supports
Catalyst tM/℃ Total acid content/(mmol·g-1 cat)* Peak fraction/% Ⅰ Ⅱ Ⅲ Ⅰ Ⅱ Ⅲ 5NiSn/SiO2 187 245 358 0.30 34.1 52.7 13.2 5NiSn/Al2O3 175 231 328 0.50 21.7 31.8 46.5 5NiSn/MgAl2O4 184 243 334 0.31 22.8 50.9 26.3 5NiSn/MgO 176 262 365 0.08 45.2 32.8 22.0 *: the amount of acid is calculated as the amount of ammonia adsorbed per gram of catalyst
tM: the temperature of the highest desorption peak -
[1] 沈菊华.国内外丙烯生产发展概况[J].化工科技市场, 2005, 28(11): 15-19. http://d.wanfangdata.com.cn/Periodical/hgkjsc200511004SHEN Ju-hua. Development of propene production at home and abroad[J]. Chem Technol Market, 2005, 28(11): 15-19. http://d.wanfangdata.com.cn/Periodical/hgkjsc200511004 [2] ZHANG Y W, ZHOU Y M, SHI J J, ZHOU S J, ZHANG Z W, ZHANG S C, GUO M G. Propane dehydrogenation over PtSnNa/La-doped Al2O3 catalyst: Effect of La content[J]. Fuel Process Technol, 2013, 111(3): 94-104. http://www.sciencedirect.com/science/article/pii/S0378382013000556 [3] ZANGENEH F T, SAHEBDELFAR S, BAHMANI M. Propane dehydrogenation over a commercial Pt-Sn/A12O3 catalyst for isobutane dehydrogenation: Optimization of reaction conditions[J]. Chin J Chem Eng, 2013, 21(7): 730-735. doi: 10.1016/S1004-9541(13)60537-6 [4] CHEN M, XU J, CAO Y, HE H Y, FAN K N, ZHUANG J H. Dehydrogenation of propane over In2O3-Al2O3 mixed oxide in the presence of carbon dioxide[J]. J Catal, 2010, 272(1): 101-108. doi: 10.1016/j.jcat.2010.03.007 [5] BAI L Y, ZHOU Y M, ZHANG Y W, LIU H, SHENG X L, XUE M W. Influence of the competitive adsorbates on the catalytic properties of PtSnNaMg/ZSM-5 catalysts for propane dehydrogenation[J]. Ind Eng Chem Res, 2011, 50(8): 4345-4350. doi: 10.1021/ie1018639 [6] 陈建九, 史海英, 汪泳.丙烷脱氢制乙烯工艺技术[J].精细石油化工进展, 2000, 1(12): 23-28. doi: 10.3969/j.issn.1009-8348.2000.12.007CHEN Jian-jiu, SHI Hai-ying, WANG Yong. Propane dehydrogenation process technology[J]. Prog Fine Petrochem, 2000, 1(12): 23-28. doi: 10.3969/j.issn.1009-8348.2000.12.007 [7] SAHEBDELFAR S, RAVANCHI M T, ZANGENEH F T, MEHRAZMA S, RAJABI S. Kinetic study of propane dehydrogenation and side reactions over Pt-Sn/Al2O3 catalyst[J]. Chem Eng Res Des, 2012, 90(8): 1090-1097. doi: 10.1016/j.cherd.2011.11.004 [8] 周宏中.国内外丙烯市场现状及发展趋势[J].化工技术经济, 2004, 22(9): 28-31. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hgjj200409005&dbname=CJFD&dbcode=CJFQZHOU Hong-zhong. Current situation and development trend of propene market at home and abroad[J]. Chem Technol Eco, 2004, 22(9): 28-31. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hgjj200409005&dbname=CJFD&dbcode=CJFQ [9] 张一卫, 周钰明, 邱安定, 王玉, 许艺, 吴沛成. Na对PtSn/ZSM-5催化丙烷脱氢反应性能的影响[J].物理化学学报, 2006, 22(6): 672-678. http://www.cqvip.com/Main/Detail.aspx?id=22130489ZHANG Yi-wei, ZHOU Yu-ming, QIU An-ding, WANG Yu, XU Yi, WU Pei-cheng. The effects of Na on the performance of PtSn/ZSM-5 catalyzed propane dehydrogenation[J]. Acta Phys-Chim Sin, 2006, 22(6): 672-678. http://www.cqvip.com/Main/Detail.aspx?id=22130489 [10] 马艳萍, 杨茹欣, 赵燕.丙烷催化脱氢制丙烯生产技术及工业应用[J].广东化工, 2012, 39(7): 87-87. http://d.wanfangdata.com.cn/Periodical/gdhg201207046MA Yan-ping, YANG Ru-xin, ZHAO Yan. Propane catalytic dehydrogenation production technology and industrial application[J]. Guangdong Chem Ind, 2012, 39(7): 87-87. http://d.wanfangdata.com.cn/Periodical/gdhg201207046 [11] 王红秋, 郑轶丹.丙烷脱氢生产丙烯技术进展[J].石化技术, 2011, 8(2): 63-66. http://www.cqvip.com/Main/Detail.aspx?id=38249482WANG Hong-qiu, ZHENG Yi-dan. Advances in propane dehydrogenation to produce propene[J]. Petrochem Technol, 2011, 8(2): 63-66. http://www.cqvip.com/Main/Detail.aspx?id=38249482 [12] 朱义才.丙烷脱氢制丙烯技术经济分析[J].当代石油石化, 2012, 20(8): 36-42. http://www.cqvip.com/QK/98497A/201208/44231455.htmlZHU Yi-cai. Technical economic analysis of propane dehydrogenation[J]. Petroleum Petrochem Today, 2012, 20(8): 36-42. http://www.cqvip.com/QK/98497A/201208/44231455.html [13] 盖希坤, 田原宇, 夏道宏.丙烷催化脱氢制丙烯工艺分析[J].炼油技术与工程, 2010, 40(12): 27-32. doi: 10.3969/j.issn.1002-106X.2010.12.007GAI Xi-kun, TIAN Yuan-yu, XIA Dao-hong. Analysis of propane catalytic dehydrogenation to propene[J]. Pet Refin Eng, 2010, 40(12): 27-32. doi: 10.3969/j.issn.1002-106X.2010.12.007 [14] GORRIZ O F, CADUS L E. Supported chromium oxide calatysts using metal carboxylate complexes: dehydrogenation of propane[J]. Appl Catal A: Gen, 1999, 180(1/2): 247-260. http://www.sciencedirect.com/science/article/pii/S0926860X98003445 [15] CIMINO A, CORDISHI D, DE ROSSI S, FERRARIS G, GAZZOLI D, INDOVINA V, VALIGI M. Studies on chromia zirconia catalysts Ⅲ. Propene hydrogenation[J]. J Catal, 1991, 127(2): 777-787. doi: 10.1016/0021-9517(91)90198-D [16] 谭晓琳, 马波, 张喜文, 张海娟, 李江红. Cr系丙烷脱氢催化剂研究进展[J].化工进展, 2010, 29(1): 51-57. http://d.wanfangdata.com.cn/Periodical/hgjz201001009TAN Xiao-lin, MA Bo, ZHANG Xi-wen, ZHANG Hai-juan, LI Jiang-hong. Research progress of Cr-based propane dehydrogenation catalyst[J]. Prog Chem, 2000, 29(1): 51-57. http://d.wanfangdata.com.cn/Periodical/hgjz201001009 [17] 邱安定, 范以宁.添加锡组分对Pt/ZSM-5催化剂丙烷脱氢反应性能的影响[J].燃料化学学报, 2008, 36(5): 637-640. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17317.shtmlQIU An-ding, FAN Yi-ning. Effect of adding tin component on propane dehydrogenation of Pt/ZSM-5 catalyst[J]. J Fuel Chem Technol, 2008, 36(5): 637-640. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17317.shtml [18] 董文生, 王心葵, 彭少逸. Ca对PtSn/MgAl2O4结构及丙烷脱氢性能的影响[J].分子催化, 1998, 12(3): 183-188. http://www.cqvip.com/Main/Detail.aspx?id=3155379DONG Wen-sheng, WANG Xin-kui, PENG Shao-yi. Effect of Ca on structure and propane dehydrogenation of PtSn/MgAl2O4[J]. J Mol Catal, 1998, 12(3): 183-188. http://www.cqvip.com/Main/Detail.aspx?id=3155379 [19] 孙毅飞, 李广超, 潘心頔, 黄传敬, 翁维正, 万惠霖.介孔氧化铝负载Ni-Co氧化物催化剂上丙烷氧化脱氢制丙烯[J].物理化学学报, 2012, 28(9): 2135-2140. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=wlhx201209020&dbname=CJFD&dbcode=CJFQSUN Yi-fei, LI Guang-chao, PAN Xin-di, HUANG Chuan-jing, WENG Wei-zheng, WAN Hui-lin. Oxidative dehydrogenation of propane to propene on Ni-Co oxide catalyst supported on mesoporous alumina[J]. Acta Phys-Chim Sin, 2012, 28(9): 2135-2140. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=wlhx201209020&dbname=CJFD&dbcode=CJFQ [20] 徐爱菊, 林勤, 照日格图, 张宇. NiO/高炉渣催化剂的丙烷脱氢性能[J].稀有金属材料与工程, 2009, 45(s1): 99-102.XU Ai-jiu, LIN Qin, ZHAO Ri-ge-tu, ZHANG Yu. Propane dehydrogenation of NiO/blast furnace slag catalyst[J]. Rare Metal Mater Eng, 2009, 45(s1): 99-102. [21] 梁旭, 刘艳侠, 蒋元力, 魏灵朝.硅改性氧化铝及负载镍基催化剂的制备与表征Ⅱ[J].工业催化, 2016, 24(9): 41-44. http://d.wanfangdata.com.cn/Periodical/gych201501010LIANG Xu, LIU Yan-xia, JIANG Yuan-li, WEI Ling-chao. Preparation and characterization of silicon modified alumina and nickel base catalystⅡ[J]. Ind Catal, 2016, 24(9): 41-44. http://d.wanfangdata.com.cn/Periodical/gych201501010 [22] WANG G W, LI C Y, SHAN H H. Highly efficient metal sulfide catalysts for selective dehydrogenation of isobutane to isobutene[J]. ACS Catal, 2014, 4(4): 1139-1143. doi: 10.1021/cs5000944 [23] WANG G W, MENG Z, LIU J W, LI C Y, SHAN H H. Promoting effect of sulfur addition on the catalytic performance of Ni/MgAl2O4 catalysts for isobutane dehydrogenation[J]. ACS Catal, 2013, 3(12): 2992-3001. doi: 10.1021/cs400705p [24] WANG G W, WANG H R, ZHANG H L, ZHU Q Q, LI C Y, SHAN H H. Highly selective and stable NiSn/SiO2 catalyst for isobutane dehydrogenation: Effects of Sn addition[J]. ChemCatChem, 2016, 8(19): 3137-3145. doi: 10.1002/cctc.v8.19 [25] 缪建文, 宋国华, 范以宁.不同孔道结构的氧化硅负载钒氧化物催化丙烷氧化脱氢[J].催化学报, 2009, 30(11): 1143-1149. doi: 10.3321/j.issn:0253-9837.2009.11.013MIAO Jian-wen, SONG Guo-hua, FAN Yi-ning. Oxidative dehydrogenation of propane oxidation catalyzed by silica supported vanadium oxide with different channel structure[J]. Chin J Catal, 2009, 30(11): 1143-1149. doi: 10.3321/j.issn:0253-9837.2009.11.013 [26] ZHANG Y W, ZHOU Y M, SHI J J, ZHOU S J, SHENG X L, ZHANG Z W, XIANG S M. Comparative study of bimetallic Pt-Sn catalysts supported on different supports for propane dehydrogenation[J]. J Mol Catal A: Chem, 2014, 381(1): 138-147. http://www.sciencedirect.com/science/article/pii/S1381116913003798 [27] HE S B, SUN C H, DU H H, BAI X H, WANG B. Effect of carbon addition on the Pt-Sn/γ-Al2O3 catalyst for long chain paraffin dehydrogenation to olefin[J]. Chem Eng J, 2008, 141(1): 284-289. http://www.sciencedirect.com/science/article/pii/S1385894708000119 [28] LAI Y L, HE S B, LI X R, SUN C L, SEAHAN K. Dehydrogenation of n-dodecane over Pt-Sn/Mg-Al-O catalysts: Investigating the catalyst performance while monitoring the products[J]. Appl Catal A: Gen, 2014, 469(17): 74-80. http://www.sciencedirect.com/science/article/pii/S0926860X1300584X [29] HE S B, SUN C L, YANG X, WANG B, BAI X H, BAI Z W. Characterization of coke deposited on spent catalysts for long-chain-paraffin dehydrogenation[J]. Chem Eng J, 2010, 163(3): 389-394. doi: 10.1016/j.cej.2010.07.024 [30] BRUSCHI L, MISTURE G. Adsorption within and on regularly patterned substrates[J]. J Low Temp Phys, 2009, 157(3-4): 206-220. doi: 10.1007/s10909-009-9913-z [31] MISTURE G, BRUSCHI L, LEE W. Adsorption on highly ordered porous alumina[J]. J Low Temp Phys, 2016, 185(1-2): 138-160. doi: 10.1007/s10909-016-1619-4 [32] LI X, MENG F H, CHENG Y, GAO Y, LI Z. Catalytic methanation in a slurry-bed reactor over Ni/SiO2 catalysts: Improvement by ZrO2 and β-cyclodextrin addition[J]. React Kinet Mech Catal, 2017, 122(1): 525-538. doi: 10.1007/s11144-017-1213-z [33] WANG H R, WANG H, LI L Y, LI C Y. Nature of active tin species and promoting effect of nickle in silica supported tin oxide for dehydrogenation of propane[J]. Appl Surf Sc, 2017, 407: 456-452. doi: 10.1016/j.apsusc.2017.02.216 [34] 邱安定, 李恩霞, 范以宁.载体组成对负载型PtSn/ZSM-5催化剂上丙烷脱氢反应性能的影响[J].催化学报, 2007, 28(11): 970-974. doi: 10.3321/j.issn:0253-9837.2007.11.009QIU An-ding, LI En-xia, FAN Yi-ning. Effect of carrier composition on dehydrogenation of propane over supported PtSn/ZSM-5 catalysts[J]. Chin J Catal, 2007, 28(11): 970-974. doi: 10.3321/j.issn:0253-9837.2007.11.009 [35] BALLARINI A D, ZGOLICZ P, VILELLA I M J, MIGUEL S R D, CASTRO A A, SCELZA O A. n-Butane dehydrogenation on Pt, PtSn and PtGe supported on γ-Al2O3 deposited on spheres of α-Al2O3 by washcoating[J]. Appl Catal A: Gen: 2010, 381(1-2): 83-91. doi: 10.1016/j.apcata.2010.03.053 [36] 徐军科, 李兆静, 汪吉辉, 周伟, 马建新.甲烷干重整催化剂Ni/Al2O3表面积碳表征和分析[J].物理化学学报, 2009, 25(2): 253-260. http://d.wanfangdata.com.cn/Periodical/wlhxxb200902010XU Jun-ke, LI Zhao-jing, WANG Ji-hui, ZHOU Wei, MA Jian-xin. Characterization and analysis of carbon deposition on the surface of Ni/Al2O3 by methane dry reforming catalyst[J]. Acta Phys-Chim Sin, 2009, 25(2): 253-260. http://d.wanfangdata.com.cn/Periodical/wlhxxb200902010 [37] KOBAYASHI Y, HORIGUCHI J, KOBAYASHI S, YAMAZAKI Y, OMATA K, NAGAO D, KONNO M, YAMADA M. Effect of NiO content in mesoporous NiO-Al2O3 catalysts for high pressure partial oxidation of methane to syngas[J]. Appl Catal A: Gen: 2011, 395(1-2): 129-137. doi: 10.1016/j.apcata.2011.01.034 [38] 董文生, 王心葵, 王浩静, 彭少逸. Pt-Sn/MgAl2O4催化剂的TPR和H2-TPD研究[J].催化学报, 1999, 20(5): 577-580. http://www.cqvip.com/Main/Detail.aspx?id=3661187DONG Wen-sheng, WANG Xin-kui, WANG Hao-jing, PENG Shao-yi. The study of TPR and H2-TPD over Pt-Sn/MgAl2O4catalyst[J]. Chin J Catal, 1999, 20(5): 577-580. http://www.cqvip.com/Main/Detail.aspx?id=3661187 [39] 郑良科, 徐成华, 刘建英, 刘盛余.以类水滑石为前驱体的铜镍基催化剂催化糠醛液相加氢[J].精细催化, 2010, 27(11): 1078-1085. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=jxhg201011010&dbname=CJFD&dbcode=CJFQZHENG Liang-ke, XU Cheng-hua, LIU Jian-ying, LIU Sheng-yu. Catalytic hydrogenation of furfural to liquid phase by catalytic Cu-Ni catalyst based on hydrotalcite-like precursor[J]. Fine Catal, 2010, 27(11): 1078-1085. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=jxhg201011010&dbname=CJFD&dbcode=CJFQ [40] 杨雅仙, 秦大伟, 谢辉. MgO改性Ni/γ-Al2O3催化剂用于甲烷重整制取合成气研究[J].天然气化工, 2012, 37(6): 40-43. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=trqh201206011&dbname=CJFD&dbcode=CJFQYANG Ya-xian, QIN Da-wei, XIE Hui. Study on preparation of syngas from methane reforming with MgO modified Ni/γ-Al2O3 catalyst[J]. Nat Gas Chem Ind, 2012, 37(6): 40-43. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=trqh201206011&dbname=CJFD&dbcode=CJFQ [41] 孔猛, 杨琦, 卢雯, 范浙永, 费金华, 郑小明, WHEELOCK T D.焙烧温度对Ni/MgO催化剂结构及其在甲苯二氧化碳重整反应中催化剂性能的影响[J].催化学报, 2012, 33(9): 1508-1516. http://d.wanfangdata.com.cn/Periodical/cuihuaxb201209012SUN Meng, YANG Qi, LU Wen, FAN Zhe-yong, FEI Jin-hua, ZHENG Xiao-ming, WHEELOCK T D. Effect of calcination temperature on the structure of Ni/MgO catalyst and its effect on the performance of catalyst in toluene reforming of toluene[J]. Chin J Catal, 2012, 33(9): 1508-1516. http://d.wanfangdata.com.cn/Periodical/cuihuaxb201209012 [42] KUMAR M, ABERUAGBA F, GUPTA J K, RAWAT K S, SHARMA L D, MURALI DHARA G. Temperature-programmed reduction and acidic properties of molybdenum supported on MgO-Al2O3 and their correlation with catalytic activity[J]. J Mol Catal A: Chem, 2004, 213(2): 217-223. doi: 10.1016/j.molcata.2003.12.005 -





 下载:
下载: