Preparation of ZnCr2O4-ZnO composite photocatalyst based on the hydrotalcite precursor and its performance in hydrogen production
-
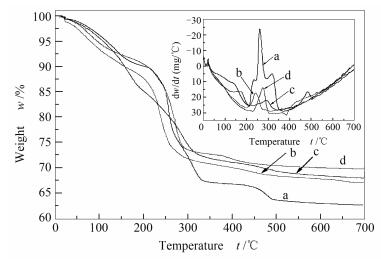
摘要: 以研磨水热法合成ZnCr2O4-ZnO异质结型光催化剂,对所得样品进行了TG-DTA、XRD、SEM、HRTEM、DRS和N2吸附-脱附表征分析;在模拟太阳光下,以草酸为牺牲剂对样品的光催化产氢活性进行评价,并分别与共沉淀法、尿素回流法和尿素水热法制备的ZnCr2O4-ZnO样品进行比较,探讨了异质结型ZnCr2O4-ZnO复合光催化剂的产氢机理。结果表明,四种方法制备的Zn-Cr前驱体都具有一定的水滑石结构,经500℃焙烧后,均为球形纳米粒子,但团聚情况各异,比表面积和孔结构参数有较大差别。其中,研磨水热法所得样品ZnCr2O4-ZnO粒子均匀,光电流响应强度最大,产氢效率最高,为0.956 mmol/(h·gcat),分别是共沉淀法、尿素回流法和尿素水热法制备样品产氢量的2.3、1.5和3.0倍。
-
关键词:
- 研磨水热法 /
- ZnCr2O4-ZnO /
- 异质结 /
- 光催化产氢
Abstract: ZnCr2O4-ZnO composite photocatalyst with heterogeneous structure was synthesized by grinding hydrothermal method and characterized by TG-DTA, XRD, SEM, HRTEM, DRS, and N2 absorption; its photocatalytic activity in H2 production was evaluated by using oxalic acid as the sacrificial agent under simulated sunlight irradiation and compared with those of the ZnCr2O4-ZnO samples prepared by coprecipitation, urea reflux and urea hydrothermal methods. The results indicate that Zn-Cr precursors prepared by four methods show a certain hydrotalcite structure; the catalyst samples prepared at 500℃ are spherical nanoparticles, but different in agglomeration status, specific surface area and pore structure parameters. The ZnCr2O4-ZnO nanoparticles prepared by a grinding hydrothermal method exhibits the optimized photocurrent response and photocatalytic activity; the yield of hydrogen production is 0.956 mmol/(h·gcat), which is 2.3, 1.5 and 3.0 times higher than that of the catalyst samples prepared by coprecipitation, urea reflux and urea hydrothermal methods, respectively. On the basis of these results, a possible mechanism for the hydrogen production over ZnCr2O4-ZnO composite photocatalyst with heterogeneous structure was then proposed. -
表 1 四种方法所得Zn-Cr-LDHs在500℃焙烧样品的比表面积和孔结构
Table 1 Specific surface area and pore-structure data of the samples prepared by four methods after calcinations at 500℃
Sample ABET / (m2·g-1) Average pore width d/nm Pore volume v/ (cm3·g-1) CP-500 81.3 18.6 0.38 UR-500 57.3 15.9 0.23 MP-500 53.3 22.8 0.31 UH-500 49.8 17.0 0.21 -
[1] FUJISHIMA K, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(7):37-38. http://www.wenkuxiazai.com/doc/2cca737302768e9951e738c2.html [2] WANG K, XU J M, WANG X T. The effects of ZnO morphology on photocatalytic efficiency of ZnO/RGO nanocomposites[J]. Appl Surf Sci, 2016, 360:270-275. doi: 10.1016/j.apsusc.2015.10.190 [3] 刘宗园, 王桂赟, 刘先平, 王延吉. CuCrO2的合成及其复合催化剂的光催化性能[J].燃料化学学报, 2013, 41(12):1473-148. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18314.shtmlLIU Zong-yuan, WANG Gui-yun, LIU Xian-ping, WANG Yan-ji. Preparation of CuCrO2 and the photocatalytic properties of its composites[J]. J Fuel Chem Technol, 2013, 41(12):1473-148. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18314.shtml [4] YU X L, AN X Q, GENC A, IBANEZ M, ARBIOL J, ZHANG Y H, and CABOT A. Cu2ZnSnS4-PtM (M=Co, Ni) Nanoheterostructures for Photocatalytic Hydrogen Evolution[J]. J Phys Chem C, 2015, 119(38):21882-21888. doi: 10.1021/acs.jpcc.5b06199 [5] 王泽岩, 黄柏标, 戴瑛.高效光催化材料的设计与制备[J].中国材料进展, 2017, 36(1):7-17. http://www.cnki.com.cn/Article/CJFDTOTAL-XJKB201701005.htmWANG Ze-yan, HUANG Bai-biao1, DAI Ying. Design and Synthesis of Highly Reactive Photocatalysts[J]. Mater China, 2017, 36(1):7-17. http://www.cnki.com.cn/Article/CJFDTOTAL-XJKB201701005.htm [6] NIKITENKO S I, CHAVE T, CAU C, BRAU H-P, FLAUD V. Photothermal Hydrogen Production Using Noble-Metal-Free Ti@TiO2 Core-Shell Nanoparticles under Visible-NIR Light Irradiation[J]. ACS Catalysis, 2015, 5(8):4790-4795. doi: 10.1021/acscatal.5b01401 [7] KLINGSHIM C. ZnO:material, physics and applications[J]. ChemPhysChem, 2007, 8(6):782-803. doi: 10.1002/(ISSN)1439-7641 [8] WANG Z L. Zinc oxide nanostructures:growth, properties and applications[J]. J Phys:Condens Matter, 2004, 16(25):R829-R858. doi: 10.1088/0953-8984/16/25/R01 [9] WANG Z, TERAMURA K, HOSOKAWA S, TANAKA T. Highly efficient photocatalytic conversion of CO2 into solid CO using H2O as a reductant over Ag-modified ZnGa2O4[J]. J Mater Chem A, 2015, 3(21):11313-11319. doi: 10.1039/C5TA01697E [10] ZHANG L, YAN J H, ZHOU M J, YANG Y H, LIU Y N. Fabrication and photocatalytic properties of spheres-in-spheres ZnO/ZnAl2O4 composite hollow microspheres[J]. Appl Surf Sci, 2013, 268:237-245. doi: 10.1016/j.apsusc.2012.12.069 [11] YANG H H, YAN J H, LU Z, CHENG X, TANG Y G. Photocatalytic activity evaluation of tetragonal CuFe2O4 nanoparticles for the H2 evolution under visible light irradiation[J]. J Alloys Compd, 2009, 476(1):715-719. http://www.academia.edu/13994341/A_review_on_the_visible_light_active_titanium_dioxide_photocatalysts_for_environmental_applications [12] GHOLAMI T, SALAVATI-NIASARI M, VARSHOY S. Investigation of the electrochemical hydrogen storage and photocatalytic properties of CoAl2O4 pigment:Green synthesis and characterization[J]. Int J Hydrogen Energy, 2016, 41(22):9418-9426. doi: 10.1016/j.ijhydene.2016.03.144 [13] PENG C, GAO L. Optical and photocatalytic properties of spinel ZnCr2O4 nanoparticles synthesized by a hydrothermal route[J]. J Am Ceram Soc, 2008, 91(7):2388-2390. doi: 10.1111/jace.2008.91.issue-7 [14] BOUMAZA S, BOUGUELIAA, BOUARAB R, TRARI M. Physical and photoelectrochemical studies for hydrogen photo-evolution over the spinel ZnCr2O4[J]. Int J Hydrogen Energy, 2009, 34(11):4963-4967. doi: 10.1016/j.ijhydene.2008.11.059 [15] KUMAR A, DIXIT T. Phase Transformation and Optical Properties of Annealed Hydrothermally Synthesized ZnO/ZnCr2O4 Nanocomposites[J]. Int J Appl Ceram. Technol, DOI:2016,10.1111/ijac.12546. [16] 祝雅杰, 吴平霄, 杨林, 陈理想, 黄柱坚. ZnO-ZnCr2O4混合金属氧化物的制备及其光催化降解双酚A[J].环境科学学报, 2016, 37(7):2428-2425. [17] PALMER S J, FROST R L, NGUYEN T. Hydrotalcites and their role in coordination of anions in Bayer liquors:anion binding in layered double hydroxides[J]. Coord Chem Rev, 2009, 253(1):250-267. https://eprints.qut.edu.au/15728/ [18] MOHAPATRA L, PARIDA K M. Zn-Cr layered double hydroxide:Visible light responsive photocatalyst for photocatalytic degradation of organic pollutants[J]. Sep Purif Technol, 2012, 91:73-80. doi: 10.1016/j.seppur.2011.10.028 [19] KIM S H, FAHEL J, DURAND PAndré E, Carteret C. Ternary layered double hydroxides (LDH) based on ZnAl substituted with Co, Cu for efficient photocatalysts designs[J]. Eur J Inorg Chem, DOI:2016,10.1002/ejic.01213. [20] POKHREL S, JEYARAJ B, NAGARAJA K S. Humidity-sensing properties of ZnCr2O4-ZnO composites[J]. Mater Lett, 2003, 57(22):3543-3548. http://www.academia.edu/2828928/Structural_and_gas-sensing_properties_of_CuO_Cu_sub_x_sub_Fe_sub_3_x_sub_O_sub_4_sub_nanostructured_thin_films [21] JIANG C, LEE K Y, PARLETT C M A, BAYAZIT M K, LAU C C, RUAN Q S, MONIZ S J A, LEE A F, TANG J W. Size-controlled TiO2 nanoparticles on porous hosts for enhanced photocatalytic hydrogen production[J]. Appl Catal, A, 2016, 521:133-139. doi: 10.1016/j.apcata.2015.12.004 [22] CHENG X, HUANG X R, WANG X Z, SUN D Z. Influence of calcination on the adsorptive removal of phosphate by Zn-Al layered double hydroxides from excess sludge liquor[J]. J Hazard Mater, 2010, 177:516-523. doi: 10.1016/j.jhazmat.2009.12.063 [23] HUO R J, KUANG Y, ZHAO Z P, ZHANG F Z, XU S L. Enhanced photocatalytic performances of hierarchical ZnO/ZnAl2O4 microsphere derived from layered double hydroxide precursor spray-dried microsphere[J]. J Colloid Interface Sci, 2013, 407:17-21. doi: 10.1016/j.jcis.2013.06.067 [24] Yu X X, Yu J G, CHENG B, JARONIEC M. Synthesis of hierarchical flower-like AlOOH and TiO2/AlOOH superstructures and their enhanced photocatalytic properties[J]. J Phys. Chem. C, 2009, 113(40):17527-17535. doi: 10.1021/jp906992r [25] GUO Q, ZHANG Z H, MA X P, JING K, SHEN M L, YU N, TANG J H, DIONYSIOU D D. Preparation of N, F-codoped TiO2 nanoparticles by three different methods and comparison of visible-light photocatalytic performances[J]. Sep Purif Technol, 2017, v175:305-313. [26] SREETHAWONG T, PUANGPETCH T, CHAVADEJ S, YOSHIKAWA S. Quantifying influence of operational parameters on photocatalytic H2 evolution over Pt-loaded nanocrystalline mesoporous TiO2 prepared by single-step sol-gel process with surfactant template[J]. J Power Sources, 2007, 165(2):861-869. doi: 10.1016/j.jpowsour.2006.12.050 [27] TAN D Z, FAN W J, XIONG W N, SUN H X, LI A, DENG W Q, MENG C G. Study on adsorption performance of conjugated microporous polymers for hydrogen and organic solvents:The role of pore volume[J]. Eur Polym J, 2012, 48(4):705-711. doi: 10.1016/j.eurpolymj.2012.01.012 [28] ZHANG Y, TANG Z R, FU X, XU Y J. Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation:what advantage does graphene have over its forebear carbon nanotube[J]. ACS Nano, 2011, 5:7426-7435. doi: 10.1021/nn202519j [29] 郝瑞鹏, 杨朋举, 王志坚, 朱珍平.贵金属负载TiO2对光催化还原CO2选择性的影响[J].燃料化学学报, 2015, 43(1):94-99. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18561.shtmlHAO Rui-peng, YANG Peng-ju, WANG Zhi-jian, ZHU Zhen-ping. Effect of noble metals loaded TiO2 on the selectivity of photocatalytic CO2 reduction[J]. J Fuel Chem Technol, 2015, 43(1):94-99. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18561.shtml [30] PARHI P, MANIVANNAN V. Microwave metathetic approach for the synthesis and characterization of ZnCr2O4[J]. J Eur Ceram Soc, 2008, 28:1665-1670. doi: 10.1016/j.jeurceramsoc.2007.11.005 [31] 张丽, 阎建辉, 周民杰, 杨亚辉, 刘又年.高比表面空心球ZnO/ZnAl2O4复合光催化剂制备及活性[J].无机化学学报, 2012, 28(9):1827-1834.ZHANG Li, YAN Jian-hui, ZHOU Min-jie, YANG Ya-hui, LIU You-nian. Preparation and Photocatalytic Property of Hollow Sphere-Like ZnO/ZnAl2O4 Composite Photocatalysts with High Specific Surface Area[J]. Chinese J Inorg Chem, 2012, 28(9):1827-1834. [32] 危阳, 马新国, 祝林, 贺华, 黄楚云.二硫化钼/石墨烯异质结的界面结合作用及其对带边电位影响的理论研究[J].物理学报, 2017, 66(8):087101-1-087101-10. http://www.cnki.com.cn/Article/CJFDTOTAL-WLXB201708031.htmWEI Yang, MA Xin-guo, ZHU Lin, HE Hua, HUANG Chu-yun. Interfacial cohesive interaction and band modulation of two-dimensional MoS2/graphene heterostructure[J]. Acta Phys Sin, 2017, 66(8):087101-1-087101-10. http://www.cnki.com.cn/Article/CJFDTOTAL-WLXB201708031.htm -





 下载:
下载: