Morphological effect of CeO2-MnOx catalyst on their catalytic performance in lean methane combustion
-
摘要: 采用水热合成法制备了船形、扁球形及纳米片CeO2-MnOx复合氧化物。并运用低温N2吸脱附、XRD、SEM、TEM、H2-TPR、拉曼光谱、XPS等表征技术对不同形貌CeO2-MnOx复合氧化物的结构与其低浓度CH4催化燃烧反应性能之间的关系进行了关联。结果表明,CeO2-MnOx复合氧化物的形貌与其催化性能密切相关。其中,扁球形CeO2-MnOx复合氧化物的氧空位、Ce3+含量及表面吸附活性氧物种最多,其CH4催化燃烧反应活性最高,540℃时,可将CH4完全转化;其次是船形CeO2-MnOx复合氧化物催化剂,540℃时其CH4转化率为94.05%;与前两者相比,纳米片CeO2-MnOx复合氧化物催化剂的氧空位及表面吸附活性氧物种较少,活性较差,相同反应温度下,其CH4转化率仅为89.68%。Abstract: Ship-like, oblate spheroid and nano sheet CeO2-MnOx mixed oxides were prepared by hydrothermal method and characterized by N2 sorption, XRD, SEM, TEM, H2-TPR, Raman spectra and XPS. The relationship between the structure of CeO2-MnOx and their catalytic performance in lean methane combustion were then investigated. The results reveal that the catalytic performance of the mixed CeO2-MnOx oxide was greatly depended on its morphology. Among all of the samples, the mixed oblate spheroid CeO2-MnOx oxides with most oxygen vacancies, Ce3+ content and surface active oxygen exhibited the highest activity in lean methane combustion, with a complete CH4 conversion at 540℃. Following it, the mixed ship-like CeO2-MnOx oxides catalyst had 94.05% of the CH4 conversion at 540℃. On the contrast, the mixed nano sheet CeO2-MnOx oxides catalyst presented the lowest catalytic activity with only 89.68% CH4 conversion at the same reaction temprature owing to its lower oxygen vacancies and surface active oxygen.
-
Key words:
- ceria /
- manganese oxide /
- lean methane /
- catalytic combustion /
- morphology
-
表 1 CeO2及不同形貌CeO2-MNOx复合氧化物的化学组成、比表面积和XRD分析
Table 1 Chemical composition, surface area and XRD analysis results of CeO2 and the mixed CeO2-MNOx oxides with different morphology
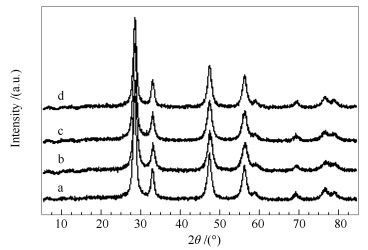
Sample Ce/Mn (molar ratio) ABET/(m2·g-1) Cell parameter a/nm a Crystallite size d/nm b CeO2 - 93.4 0.5416 19.3 CeO2-MNOx(SP) 9.0 79.5 0.5411 22.7 CeO2-MNOx(OB) 8.9 88.6 0.5402 18.6 CeO2-MNOx(NS) 8.9 90.3 0.5408 17.1 a:calculated from the a value of the ceria (111) planes;b:calculated with the scherrer equation 表 2 CeO2及不同形貌CeO2-MNOx复合氧化物催化剂的H2-TPR定量分析
Table 2 H2-TPR quantitative analysis of CeO2 and the mixed CeO2-MNOx oxides with different morphology
Sample Peak position t/℃ H2 uptake /(μmol·g-1) Theoretical H2 uptake /(μmol·g-1)a CeO2 500 1820 2904 CeO2-MNOx(SP) 240, 310, 394, 452 242, 363, 798, 605 2619 CeO2-MNOx(OB) 246, 300, 404, 448 306, 353, 848, 746 2623 CeO2-MNOx(NS) 248, 306, 400, 441 255, 423, 804, 473 2628 a:theoretical H2 uptake was determined as the quantity of H2 required for the reduction of CeO2 and the mixed CeO2-MNOx oxides by assuming that CeO2 and MNOx (as MnO2) are stoichiometrically reduced to Ce2O3 and MnO, respectively 表 3 CeO2及不同形貌CeO2-MNOx复合氧化物催化剂的XPS分析
Table 3 XPS analysis results of CeO2 and the mixed CeO2-MNOx oxides with different morphology
Sample Content w/% Ce3+ Mn4+ Mn3+ Mn2+ O″ CeO2 17.11 - - - 18.49 CeO2-MNOx(SP) 19.19 20.84 27.16 52.00 34.88 CeO2-MNOx(OB) 20.37 10.24 28.10 61.66 55.87 CeO2-MNOx(NS) 18.25 34.15 15.97 49.88 28.47 -
[1] SU S, BEATH A, GUO H, MALLETT C. An assessment of mine methane mitigation and utilisation technologies[J]. Prog Energy Combust Sci, 2005, 31(2):123-170. doi: 10.1016/j.pecs.2004.11.001 [2] DEBBAGH M N, LECEA C S M D, PÉREZ-RAMÍREZ J. Catalytic reduction of N2O over steam-activated FeZSM-5 zeolite:Comparison of CH4, CO, and their mixtures as reductants with or without excess O2[J]. Appl Catal B:Environ, 2007, 70(1/4):334-341. [3] LUO J J, XU H Y, LIU Y F, CHU W, JIANG C F, ZHAO X S. A facile approach for the preparation of biomorphic CuO-ZrO2 catalyst for catalytic combustion of methane[J]. Appl Catal A:Gen, 2012, 423/424:121-129. doi: 10.1016/j.apcata.2012.02.025 [4] 张佳瑾, 李建伟, 朱吉钦, 王越, 陈标华.助剂对Cu-Mn复合氧化物整体式催化剂催化低浓度甲烷燃烧反应性能的影响[J].催化学报, 2011, 32(8):1380-1386. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201108012ZHANG Jia-jin, LI Jian-wei, ZHU Ji-qin, WANG Yue, CHEN Biao-hua. Effect of promoter on the performance of Cu-Mn complex oxide monolithic catalysts for lean methane catalytic combustion[J]. Chin J Catal, 2011, 32(8):1380-1386. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201108012 [5] SI R, FLYTZANI-STEPHANOPOULOS M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water-gas shift reaction[J]. Angew Chem Int Ed, 2008, 47(15):2884-2887. doi: 10.1002/(ISSN)1521-3773 [6] TANG X F, CHEN J L, LI Y G, LI Y, XU Y D, SHEN W J. Complete oxidation of formaldehyde over Ag/MnOx-CeO2 catalysts[J]. Chem Eng J, 2006, 118(1/2):119-125. https://www.sciencedirect.com/science/article/pii/S0022328X12000162 [7] LI H J, QI G, TA N, ZHANG X J, LI W, SHEN W J. Morphological impact of manganese-cerium oxides on ethanol oxidation[J]. Catal Sci Technol, 2011, 1(9):1677-1682. doi: 10.1039/c1cy00308a [8] WANG X Y, KANG Q, LI D. Low-temperature catalytic combustion of chlorobenzene over MnOx-CeO2 mixed oxide catalysts[J]. Catal Commun, 2008, 9(13):2158-2162. doi: 10.1016/j.catcom.2008.04.021 [9] LI J, ZHU P F, ZHOU R X. Effect of the preparation method on the performance of CuO-MnOx-CeO2 catalysts for selective oxidation of CO in H2-rich streams[J]. J Power Sources, 2011, 196(22):9590-9598. doi: 10.1016/j.jpowsour.2011.07.052 [10] ZHANG Y G, QIN Z F, WANG G F, ZHU H Q, DONG M, LI S N, WU Z W, LI Z K, WU Z H, ZHANG J, HU T D, FAN W B, WANG J G. Catalytic performance of MnOx-NiO composite oxide in lean methane combustion at low temperature[J]. Appl Catal B:Environ, 2013, 129(2):172-181. http://downloads.hindawi.com/journals/jchem/2017/2937108.xml [11] LI S N, ZHU H Q, QIN Z F, WANG G F, ZHANG Y G, WU Z W, LI Z K, CHEN G, DONG W W, WU Z H, ZHENG L R, ZHANG J, HU T D, WANG J G. Morphologic effects of nano CeO2-TiO2 on the performance of Au/CeO2-TiO2 catalysts in low-temperature CO oxidation[J]. Appl Catal B:Environ, 2014, 144(2):498-506. [12] MA C J, WEN Y Y, YUE Q Q, LI A Q, ZHANG N W, GAI H J, ZHENG J B, CHEN B H. Oxygen-vacancy-promoted catalytic wet air oxidation of phenol from MnOx-CeO2[J]. RSC Adv, 2017, 7(43):27079-27088. doi: 10.1039/C7RA04037G [13] ZHANG P F, LU H F, ZHOU Y, ZHANG L, WU Z L, YANG S Z, SHI H L, ZHU Q L, CHEN Y F, DAI S. Mesoporous MnCeOx solid solutions for low temperature and selective oxidation of hydrocarbons[J]. Nat Commun, 2015, 6:8448-8458. doi: 10.1038/ncomms9448 [14] TANG X F, LI Y G, HUANG X M, XU Y D, ZHU H Q, WANG J G, SHEN W J. MnOx-CeO2 mixed oxide catalysts for complete oxidation of formaldehyde:Effect of preparation method and calcination temperature[J]. Appl Catal B:Environ, 2006, 62(3/4):265-273. http://downloads.hindawi.com/journals/jchem/2017/2937108.xml [15] CEN W L, LIU Y, WU Z B, WANG H Q, WENG X L. A theoretic insight into the catalytic activity promotion of CeO2 surfaces by Mn doping[J]. Phys Chem Chem Phys, 2012, 14(16):5769-5777. doi: 10.1039/c2cp00061j [16] FRANCISCO M S P, MASTELARO V R, NASCENTE P A P, FLORENTINO A O. Activity and characterization by XPS, HR-TEM, Raman spectroscopy, and BET surface area of CuO/CeO2-TiO2 catalysts[J]. J Phys Chem B, 2001, 105(43):10515-10522. doi: 10.1021/jp0109675 [17] 李树娜, 石奇, 李小军, 方振华, 孙平, 周跃花, 张杏梅, 杨晓慧.金属掺杂Ce-M(M=Fe、Ni和Cu)催化剂的CO低温氧化性能研究[J].燃料化学学报, 2017, 45(6):707-713. http://www.cjcatal.org/CN/abstract/abstract21892.shtmlLI Shu-na, SHI Qi, LI Xiao-jun, FANG Zhen-hua, SUN Ping, ZHOU Yue-hua, ZHANG Xing-mei, YANG Xiao-hui. Low temperature CO oxidation over the ceria oxide catalysts doped with Fe, Ni and Cu[J]. J Fuel Chem Technol, 2017, 45(6):707-713. http://www.cjcatal.org/CN/abstract/abstract21892.shtml [18] 李岚, 胡庚申, 鲁继青, 罗孟飞. CeO2基固溶体氧缺位拉曼光谱表征的研究进展[J].物理化学学报, 2012, 28(5):1012-1020. doi: 10.3866/PKU.WHXB201203052LI Lan, HU Geng-shen, LU Ji-qing, LUO Meng-fei. Review of oxygen vacancies in CeO2-doped solid solutions as characterized by Raman spectroscopy[J]. Acta Phys-Chim Sin, 2012, 28(5):1012-1020. doi: 10.3866/PKU.WHXB201203052 [19] HERNÁNDEZ W Y, CENTENO M A, ROMERO-SARRIA F, ODRIOZOLA J A. Synthesis and characterization of Ce1-xEuxO2-x/2 mixed oxides and their catalytic activities for CO oxidation[J]. J Phys Chem C, 2009, 113(14):5629-5635. doi: 10.1021/jp8092989 [20] HUA G M, ZHANG L D, FEI G T, FANG M. Enhanced catalytic activity induced by defects in mesoporous ceria nanotubes[J]. J Mater Chem, 2012, 22(14):6851-6855. doi: 10.1039/c2jm13610d [21] BÊCHE E, CHARVIN P, PERARNAU D, ABANADES S, FLAMANT G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz)[J]. Surf Interface Anal, 2008, 40(3/4):264-267. doi: 10.1002/sia.2686/full®ionCode=US-WA&identityKey=1bf82d9d-3013-4526-8c2f-11ee6f8097ab [22] LI S N, ZHANG Y G, LI X J, YANG X H, LI Z K, WANG R Y, ZHU H Q. Preferential oxidation of CO in H2-rich stream over Au/CeO2-NiO catalysts:Effect of the preparation method[J]. Catal Lett, 2018, 148(1):328-340. doi: 10.1007/s10562-017-2231-1 [23] SKORODUMOVA N V, SIMAK S I, LUNDQVIST B I, ABRIKOSOV I A, JOHANSSON B. Quantum origin of the oxygen storage capability of ceria[J]. Phys Rev Lett, 2002, 89(16):166601-1166601-4. doi: 10.1103/PhysRevLett.89.166601 [24] 袁善良, 兰海, 薄其飞, 张彪, 肖熙, 蒋毅. TiO2掺杂CuMnCe/Al2O3催化剂对甲烷催化燃烧脱氧反应的影响[J].燃料化学学报, 2017, 45(2):243-248. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201702015&dbname=CJFD&dbcode=CJFQYUAN Shan-liang, LAN Hai, BO Qi-fei, ZHANG Biao, XIAO Xi, JIANG Yi. Effect of TiO2 doping on methane catalytic combustion deoxidation of CuMnCe/Al2O3 catalyst[J]. J Fuel Chem Technol, 2017, 45(2):243-248. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201702015&dbname=CJFD&dbcode=CJFQ [25] HAMOUDI S, LARACHI F, ADNOT A, SAYARI A. Characterization of spent MnO2/CeO2 wet oxidation catalyst by TPO-MS, XPS, and S-SIMS[J]. J Catal, 1999, 185(2):333-334. doi: 10.1006/jcat.1999.2519 [26] DELIMARIS D, IOANNIDES T. VOC oxidation over MnOx-CeO2 catalysts prepared by a combustion method[J]. Appl Catal B:Environ, 2008, 84(1/2):303-312. http://downloads.hindawi.com/journals/jchem/2017/2937108.xml [27] ZHAO P, WANG C N, HE F, LIU S T. Effect of ceria morphology on the activity of MnOx/CeO2 catalysts for the catalytic combustion of chlorobenzene[J]. RSC Adv, 2014, 4(86):45665-45672. doi: 10.1039/C4RA07843H [28] ŚWIATOWSKA J, LAIR V, PEREIRA-NABAIS C, COTE G, MARCUS P, CHAGNES A. XPS, XRD and SEM characterization of a thin ceria layer deposited onto graphite electrode for application in lithium-ion batteries[J]. Appl Surf Sci, 2011, 257(21):9110-9119. doi: 10.1016/j.apsusc.2011.05.108 [29] TROVARELLI A. Catalytic properties of ceria and CeO-containing materials[J]. Catal Rev, 1996, 38(4):439-520. doi: 10.1080/01614949608006464 [30] LU H, KONG X, HUANG H, ZHOU Y, CHEN Y. Cu-Mn-Ce ternary mixed-oxide catalysts for catalytic combustion of toluene[J]. J Environ Sci, 2015, 32(6):102-107. https://www.sciencedirect.com/science/article/pii/S1001074215001369 -





 下载:
下载: