Effect of phase transformation of La2Zr2O7 catalysts on catalytic performance for the oxidative coupling of methane
-
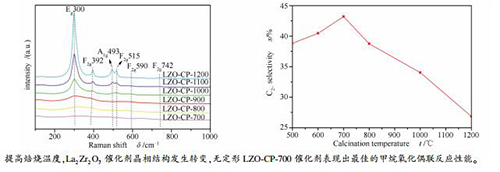
摘要: 采用共沉淀法并通过改变焙烧温度制备了一系列具有不同晶相结构的La2Zr2O7催化剂,在微型固定床反应器上评价其甲烷氧化偶联反应性能,并利用XRD、Raman、CO2-TPD、XPS等表征手段,探究催化剂的物相结构、表面碱性以及表面氧物种的变化规律。结果表明,随着焙烧温度从700℃逐渐升高到1200℃,La2Zr2O7催化剂结晶度不断提高,晶相发生明显变化,从无定形结构逐渐向缺陷萤石结构过渡,最终转变成烧绿石结构。焙烧温度提高促使La2Zr2O7晶相转变过程中,催化剂表面的碱性强度减弱,中等碱性位数量以及具有催化活性的表面氧物种O22-和O2-的相对含量不断减少,致使催化剂的CH4转化率和C2+选择性不断降低。其中,无定形LZO-CP-700催化剂表现出最佳的甲烷氧化偶联反应性能。Abstract: La2Zr2O7 catalyst was prepared using co-precipitation method and then calcined at different temperatures to obtain a series of catalysts with different phase structures. Their catalytic performances for oxidative coupling of methane were evaluated in a fixed bed micro-reactor. Meanwhile, the changes of phase structure, surface base sites and surface oxygen species upon these samples were characterized with XRD, Raman, CO2-TPD, and XPS. With the increase of the calcination temperature from 700 to 1200℃, the crystallinity of La2Zr2O7 catalysts increased continuously and the crystal phase changed obviously. The structure of the catalysts gradually changed from amorphous to disordered defective fluorite structure, and ultimately to phrochlore structure. Both the number of medium basic sites and the electrophilic oxygen species, such as O22- and O2-, on the catalysts decreased with the phase transition caused by the temperature raising, resulting in the decrease of CH4 conversion and C2+ selectivity. The amorphous LZO-CP-700 catalyst showed the best performance in methane oxidation coupling reaction.
-
Key words:
- La2Zr2O7 /
- structural change /
- surface base /
- oxygen species /
- oxidative coupling of methane
-
表 1 La2Zr2O7催化剂的BET表征
Table 1 BET results of the La2Zr2O7 catalysts
Sample SBET /(m2·g-1) LZO-CP-700 2.3 LZO-CP-800 1.8 LZO-CP-1000 1.5 LZO-CP-1200 0.7 表 2 催化剂CO2-TPD的量化
Table 2 Quantified CO2-TPD results of the La2Zr2O7 catalysts
Sample Weak basic sites amount /(μmol·g-1) Intermediate basic sites amount /(μmol·g-1) Total basic sites amount/(μmol·g-1) LZO-CP-700 47 251 298 LZO-CP-800 30 194 224 LZO-CP-900 18 113 131 LZO-CP-1000 15 48 63 LZO-CP-1100 12 15 27 LZO-CP-1200 6 6 12 表 3 La2Zr2O7催化剂的XPS拟合
Table 3 XPS O 1s peaks fitting of the La2Zr2O7 catalysts
Sample Relative amount /%a (O2-+ O22-)/O2- O2- CO32- O22- O2- LZO-CP-700 11.1 22.4 37.4 29.0 1.67 LZO-CP-800 25.4 24.9 18.8 30.9 1.44 LZO-CP-1000 26.0 28.0 15.3 30.7 1.35 LZO-CP-1200 7.9 24.5 19.0 48.5 0.55 a: percentage of oxygen species on the surface of the catalyst 表 4 La2Zr2O7催化剂的O2-TPD定量
Table 4 Quantified O2-TPD results of the La2Zr2O7 catalysts
Sample Oxygen amount of 100-200 ℃ Oxygen amount of 200-400 ℃ Oxygen amount of above 400 ℃ Total oxygen amount /(a.u.) LZO-CP-700 2 75 23 100 LZO-CP-800 1 36 20 57 LZO-CP-1000 1 15 14 30 LZO-CP-1200 1 10 0 11 note: relative value of total oxygen species on the surface of LZO-CP-700 catalyst is 100 for calculation and comparison -
[1] CRUELLAS A, BAKKER J J, VAN SINT ANNALAND M, MEDRANO J A, GALLUCCI F. Techno-economic analysis of oxidative coupling of methane:Current state of the art and future perspectives[J]. Energy Convers Manage, 2019, 198. http://cn.bing.com/academic/profile?id=1114df4758338640fdecbff1b131687b&encoded=0&v=paper_preview&mkt=zh-cn [2] 胡徐腾.天然气制乙烯技术进展及经济性分析[J].化工进展, 2016, 35(6):1733-1738. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201606013HU Teng-fei. Technological progress and economic analysis of ethylene production from natural gas[J]. Chem Ind Eng Prog, 2016, 35(6):1733-1738. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201606013 [3] 张明森, 冯英杰, 柯丽, 武洁花, 赵清锐.甲烷氧化偶联制乙烯催化剂的研究进展[J].石油化工, 2015, 44(4):401-408. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhg201504001ZHANG Ming-sen, FENG Yingjie, KE Li, WU Jie-hua, ZHAO Qingrui. Advances in the study of catalysts for the oxidation of methane to ethylene[J]. Petrochem Technol (China), 2015, 44(4):401-408. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhg201504001 [4] OH S C, WU Y, TRAN D T, LEE I C, LEI Y, LIU D. Influences of cation and anion substitutions on oxidative coupling of methane over hydroxyapatite catalysts[J]. Fuel, 2016, 167:208-217. doi: 10.1016/j.fuel.2015.11.058 [5] KWON D, YANG I, SIM Y, HA J M, JUNG J C. A K2NiF4-type La2Li0.5Al0.5O4 catalyst for the oxidative coupling of methane (OCM)[J]. Catal Commun, 2019, 128:1-5. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d9eb90e6f22ba7d6b2b67554ecdc8659 [6] PAK S, LUNSFORD J H. Thermal effects during the oxidative coupling of methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO catalysts[J]. Appl Catal A:Gen, 1998, 168(1):131-137. doi: 10.1016/S0926-860X(97)00340-2 [7] 李鹏, 张明森, 武洁花.甲烷氧化偶联制乙烯机理和动力学研究进展[J].石油化工, 2018, 47(9):1005-1012. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhg201809017Li Peng, ZHANG Ming-sen, WU Jie-hua. Research progress on mechanism and kinetics of methane oxidation coupling to ethylene production[J]. Petrochem Technol (China), 2018, 47(9):1005-1012. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhg201809017 [8] GAMBO Y, JALIL A A, TRIWAHYONO S, ABDULRASHEED A A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene:A review[J]. J Ind Eng Chem, 2018, 59:218-229. doi: 10.1016/j.jiec.2017.10.027 [9] KIM I, LEE G, NA H B, HA J M, JUNG J C. Selective oxygen species for the oxidative coupling of methane[J]. Mol Catal, 2017, 435:13-23. doi: 10.1016/j.mcat.2017.03.012 [10] 唐新德, 叶红齐, 马晨霞, 刘辉.烧绿石型复合氧化物的结构、制备及其光催化性能[J].化学进展, 2009, 21(10):2100-2114. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz200910014TANG Xin-de, YE Hong-qi, MA Chen-xia, LIU Hui. Structure, preparation and photocatalytic properties of pyroclastic oxide[J]. Chem Ind Eng Prog, 2009, 21(10):2100-2114. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz200910014 [11] XU J, ZHANG Y, XU X, FANG X, XI R, LIU Y, ZHENG R, WANG X. Constructing La2B2O7 (B=Ti, Zr, Ce) compounds with three typical crystalline phases for the oxidative coupling of methane:The effect of phase structures, superoxide anions, and alkalinity on the reactivity[J]. ACS Catal, 2019, 9(5):4030-4045. doi: 10.1021/acscatal.9b00022 [12] MINERVINI L G R W, SICKAFUS K E. Disorder in pyrochlore oxides[J]. J Am Ceram Soc, 2000, 83(8):1873-1878. http://cn.bing.com/academic/profile?id=0eb3aaa346c4d69f77fa27adde90b8e9&encoded=0&v=paper_preview&mkt=zh-cn [13] LANG M, ZHANG F, ZHANG J, WANG J, LIAN J, WEBER W J, SCHUSTER B, TRAUTMANN C, NEUMANN R, EWING R C. Review of A2B2O7 pyrochlore response to irradiation and pressure[J]. Nucl Instrum Methods Phys Ressect B, 2010, 268(19):2951-2959. doi: 10.1016/j.nimb.2010.05.016 [14] SHAFIQUE M, KENNEDY B J, IQBAL Y, UBIC R. The effect of B-site substitution on structural transformation and ionic conductivity in Ho2(ZryTi1-y)(2)O7[J]. J Alloy Compd, 2016, 671:226-233. doi: 10.1016/j.jallcom.2016.02.087 [15] ASHCROFT A T, CHEETHAM A K, GREEN M L H, GREY C P, VERNON P D F. Oxidative coupling of methane over tin-containing rare-earth pyrochlores[J]. J Chem Soc:Chem Commun, 1989(21):1667-1669. doi: 10.1039/c39890001667 [16] PETIT C, REHSPRINGER J L, KADDOURI A, LIBS S, POIX P, KIENNEMANN A. Bond energy effects in methane oxidative coupling on phrochlore structures[J]. J Catal, 1993, 140:328-334. doi: 10.1006/jcat.1993.1087 [17] XU J, PENG L, FANG X, FU Z, LIU W, XU X, PENG H, ZHENG R, WANG X. Developing reactive catalysts for low temperature oxidative coupling of methane:On the factors deciding the reaction performance of Ln2Ce2O7 with different rare earth A sites[J]. Appl Catal A:Gen, 2018, 552:117-128. doi: 10.1016/j.apcata.2018.01.004 [18] FANG X, XIA L, PENG L, LUO Y, XU J, XU L, XU X, LIU W, ZHENG R, WANG X. Ln2Zr2O7 compounds (Ln=La, Pr, Sm, Y) with varied rare earth A sites for low temperature oxidative coupling of methane[J]. Chin Chem Lett, 2019, 30(6):1141-1146. doi: 10.1016/j.cclet.2019.03.031 [19] CHEN H, GAO Y, LIU Y, LUO H. Coprecipitation synthesis and thermal conductivity of La2Zr2O7[J]. J Alloy Compd, 2009, 480(2):843-848. doi: 10.1016/j.jallcom.2009.02.081 [20] KONG L, KARATCHEVTSEVA I, GREGG D J, BLACKFORD M G, HOLMES R, TRIANI G, VANDERAH T. A novel chemical route to prepare La2Zr2O7 pyrochlore[J]. J Am Ceram Soc, 2013, 96(3):935-941. doi: 10.1111/jace.12060 [21] TONG Y, LU L, YANG X, WANG X. Characterization and their photocatalytic properties of Ln2Zr2O7 (Ln=La, Nd, Sm, Dy, Er) nanocrystals by stearic acid method[J]. Solid State Sci, 2008, 10(10):1379-1383. doi: 10.1016/j.solidstatesciences.2008.01.027 [22] CHARTIER A, MEIS C, WEBER W J, CORRALES L R. Theoretical study of disorder in Ti-substituted La2Zr2O7[J]. Phys Rev B, 2002, 65(13). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=426f4bf770e1f5f287598f8e12a8a88a [23] WILDE P J, CATLOW C R A. Defects and diffusion in pyrochlore structured oxides[J]. Solid State Ion, 1998, 112(3/4):173-183. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e27cdf5a68838cc1f49b640705f789b4 [24] MICHEL D, PEREZYJORBA M, COLLONGUES R. Study by Raman-spectroscopy of order-disorder phenomena occurring in some binary oxides with fluorite-related structures[J]. J Raman Spectrosc, 1976, 5(2):163-180. doi: 10.1002/jrs.1250050208 [25] 王烈林, 谢华, 江阔, 邓超, 龙勇, 康晓庆, 米国源.喷雾热解合成An2Zr2O7(An=La、Nd)烧绿石及结构分析[J].核化学与放射化学, 2014, 36(4):241-246. http://d.wanfangdata.com.cn/Periodical/hhxyfshx201404009WANG Lie-lin, XIE Hua, JIANG Kuo, DENG Chao, LONG Yong, KANG Xiao-qing, MI Guo-yuan. Synthesis and structure analysis of pyrochlore An_2Zr_2O_7(An=La、Nd) by spray pyrolysis[J]. J Nucl Radiochem, 2014, 36(4):241-246. http://d.wanfangdata.com.cn/Periodical/hhxyfshx201404009 [26] DUBOIS J L, CAMERON C J. Common features of oxidative coupling of methane cofeed catalysts[J]. Appl Catal, 1990, 67(1):49-71. http://cn.bing.com/academic/profile?id=5b382579c57556c39af5efbffca40940&encoded=0&v=paper_preview&mkt=zh-cn [27] CHOUDHARY V R, RANE V H. Acidity basicity of rare-earth-oxides and their catalytic activity in oxidative coupling of methane to C2-hydrocarbons[J]. J Catal, 1991, 130(2):411-422. http://cn.bing.com/academic/profile?id=82ca34335ee8f54c03477063f2925659&encoded=0&v=paper_preview&mkt=zh-cn [28] PAPA F, LUMINITA P, OSICEANU P, BIRJEGA R, AKANE M, BALINT I. Acid-base properties of the active sites responsible for C2+ and CO2 formation over MO-Sm2O3 (M=Zn, Mg, Ca and Sr) mixed oxides in OCM reaction[J]. J Mol Catal A:Chem, 2011, 346(1/2):46-54. [29] PENG L, XU J, FANG X, LIU W, XU X, LIU L, LI Z, PENG H, ZHENG R, WANG X. SnO2 Based catalysts with low-temperature performance for oxidative coupling of methane:Insight into the promotional effects of alkali-metal oxides[J]. Eur J Inorg Chem, 2018, 2018(17):1787-1799. doi: 10.1002/ejic.201701440 [30] ELKINS T W, ROBERTS S J, HAGELIN-WEAVER H E. Effects of alkali and alkaline-earth metal dopants on magnesium oxide supported rare-earth oxide catalysts in the oxidative coupling of methane[J]. Appl Catal A:Gen, 2016, 528:175-190. doi: 10.1016/j.apcata.2016.09.011 [31] FERREIRA V J, TAVARES P, FIGUEIREDO J L, FARIA J L. Ce-Doped La2O3 based catalyst for the oxidative coupling of methane[J]. Catal Commun, 2013, 42:50-53. doi: 10.1016/j.catcom.2013.07.035 [32] ZHANG Y, XU J, XU X, XI R, LIU Y, FANG X, WANG X. Tailoring La2Ce2O7 catalysts for low temperature oxidative coupling of methane by optimizing the preparation methods[J]. Catal Today, 2019. http://www.researchgate.net/publication/333914321_Tailoring_La2Ce2O7_catalysts_for_low_temperature_oxidative_coupling_of_methane_by_optimizing_the_preparation_methods -





 下载:
下载: