In situ XRD study of the effect of H2O on Fe5C2 phase and Fischer-Tropsch performance
-
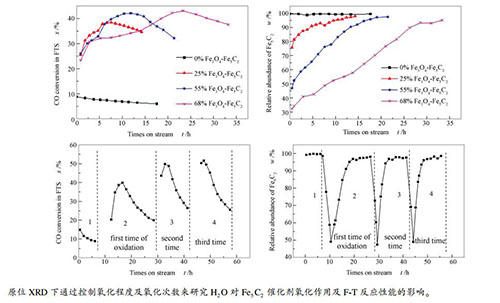
摘要: 将原位XRD反应装置与在线气相色谱技术结合,研究了不同H2O含量(4.36%、1.68%、0.56%)条件下单一相Fe5C2的氧化速率,并考察了不同Fe5C2氧化程度(0、25%、55%、68%)和氧化次数对其费托合成(F-T)反应性能的影响。研究结果表明,Fe5C2物相的氧化速率随H2O含量的提高而逐渐增加,同时H2O氧化使Fe5C2颗粒粒径减小,暴露出更多活性位点,造成F-T反应活性提高,且氧化程度越大,活性提高越明显;随着氧化次数的增加,F-T反应活性逐渐提高,但CH4选择性增加,C5+选择性逐渐降低。Abstract: In situ XRD reaction device combined with the online gas chromatography was used to study the oxidation behavior of the effect of H2O content (4.36%, 1.68%, 0.56%) on the phase and Fischer-Tropsch synthesis (FTS) performance of the single phase Fe5C2. The results show that the oxidation rate of the Fe5C2 phase increases with the increase of the content of injected H2O. Meanwhile, the particle size of Fe5C2 phase decreases and more active sites exposes during the H2O oxidation, resulting in the increase of the FTS activity. Furthermore, the FTS activity increases with the increase of the oxidation times, but the selectivity of CH4 increases and the C5+ selectivity decreases gradually.
-
Key words:
- Fe5C2 /
- H2O oxidation /
- in situ XRD /
- Fischer-Tropsch synthesis
-
图 1 原位XRD反应装置示意图
Figure 1 Schematic diagram of the in situ XRD reactor
1: normal gas cylinder; 2: needle valve; 3: pressure reducing valve; 4: mass flow controller; 5: check valve; 6: bubbler; 7: in situ capillary reactor setup (a: thermocouple, b: silica capillary, c: catalyst sample, d: silica wool); 8: back pressure valve; 9: gas chromatography (agilent 6890 N)
-
[1] 戴德立. 2018BP世界能源统计年鉴[Z].http://www.bp.Com/papercopies. 2018-6.DUDLEY B. BP Statistical Review of World Energy[Z]. http://www.bp.com/papercopies. 2018-6. [2] RÖPER M. Fischer-Tropsch Synthesis[C]// Catalysis in C 1 Chemistry. 1983. [3] ANDERSO R B, KOLBE H, RALEK M. The Fischer-Tropsch Synthesis[M]. NewYork: Academic Press, 1984. [4] 温晓东, 杨勇, 相宏伟, 焦海军, 李永旺.费托合成铁基催化剂的设计基础:从理论走向实践[J].中国科学:化学, 2017, 47(11): 1298-1311. http://www.cnki.com.cn/Article/CJFDTotal-JBXK201711007.htmWEN Xiao-dong, YANG Yong, XIANG Hong-wei, JIAO Hai-jun, LI Yong-wang. Design basis of fischer-tropsch synthesis of iron-based catalysts: from theory to practice[J]. Chin Sci: Chem, 2017, 47(11): 1298-1311. http://www.cnki.com.cn/Article/CJFDTotal-JBXK201711007.htm [5] WANG Y, KANG J, ZHANG Q. Research advances in catalysts for fischer-tropsch synthesis[J]. Pet Technol, 2009, 38(12): 1255-1263. [6] SMIT E D, WECKHUYSEN B M. ChemInform abstract: The renaissance of iron-based Fischer-Tropsch synthesis: The multifaceted catalyst deactivation behavior[J]. ChemInform, 2010, 40(19): 2758-2781. http://www.researchgate.net/publication/250483564_ChemInform_Abstract_The_Renaissance_of_Iron-Based_Fischer-Tropsch_Synthesis_The_Multifaceted_Catalyst_Deactivation_Behavior [7] LI S, ROBERT J O, MEITZNER G D. Structural analysis of unpromoted Fe-based Fischer-Tropsch catalysts using X-ray absorption spectroscopy[J]. Appl Catal A: Gen, 2001, 219(1): 215-222. doi: 10.1016-S0926-860X(01)00694-9/ [8] DUVENHAGE D J, ESPINOZA R L, COVILLE N J. fischer-tropsch precipitated iron catalysts: Deactivation studies[J]. Stud Surf Sci Catal, 1994, 88: 351-358. doi: 10.1016/S0167-2991(08)62760-3 [9] BUKUR D B, OKABE K, ROSYNEK M P. Activation studies with a precipitated iron catalyst for fischer-tropsch synthesis. Ⅰ: Characterization studies[J]. J Catal, 1995, 155(2): 353-365. doi: 10.1006/jcat.1995.1217 [10] BARTHOLOMEW C H, STOKER M W, MANSKER L. Effects of pretreatment, reaction, and promoter on microphase structure and fischer-tropsch activity of precipitated iron catalysts[J]. Stud Surf Sci Catal, 1999, 126: 265-272. doi: 10.1016/S0167-2991(99)80475-3 [11] REYMOND J P, MERIAUDEAU P, TEICHNER S J. Changes in the surface structure and composition of an iron catalyst of reduced or unreduced Fe2O3 during the reaction of carbon monoxide and hydrogen[J]. J Catal, 1982, 75(1): 39-48. http://www.sciencedirect.com/science/article/pii/0021951782901191 [12] BUTT J B. Carbide phases on iron-based fischer-tropsch synthesis catalysts part Ⅰ: Characterization studies[J]. Catal Lett, 1990, 7(1/4): 61-81. doi: 10.1007/BF00764492 [13] RAUPP G B, DELGASS W N. Mössbauer investigation of supported Fe and FeNi catalysts: Ⅱ. Carbides formed fischer-tropsch synthesis[J]. J Catal, 1979, 58(3): 348-360. doi: 10.1016/0021-9517(79)90274-4 [14] MACHOCKI K. Formation of carbonaceous deposit and its effect on carbon monoxide hydrogenation on iron-based catalysts[J]. Appl Catal: Gen, 1991, 70(1): 237-252. doi: 10.1016/S0166-9834(00)84167-6 [15] DWYER D J, HARDENBERGH J H. The catalytic reduction of carbon monoxide over iron surfaces: A surface science investigation[J]. Chem Inform, 1984, 87(1): 66-76. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1177/096032719601500807 [16] DRY M E. Catalysis-Science and Technology[M]. NewYork: Springer Verlag, 1988: 160-255. [17] MANSKER L D, JIN Y, BUKUR D B. Characterization of slurry phase iron catalysts for fischer-tropsch synthesis[J]. Appl Catal A: Gen, 1999, 186(s 1/2): 277-296. http://www.sciencedirect.com/science/article/pii/S0926860X99001490 [18] WELLER S, HOFER L J E, ANDERSON R B. The role of bulk cobalt carbide in the Fischer-Tropsch synthesis1[J]. J Am Chem Soc, 1948(2): 799-801. http://www.researchgate.net/publication/231491002_The_Role_of_Bulk_Cobalt_Carbide_in_the_FischerTropsch_Synthesis1 [19] BUKUR D B, NOWICKI L, MANNE R K. Activation studies with a precipitated iron catalyst for Fischer-Tropsch synthesis: Ⅱ. Reaction studies[J]. J Catal, 1995, 155(2): 366-375. doi: 10.1006/jcat.1995.1218 [20] BARTHOLOMEW C H, BOWMAN R M. Sulfur poisoning of cobalt and iron Fischer-Tropsch catalysts[J]. Appl Catal A: Gen, 1985, 15(1): 59-67. doi: 10.1016/S0166-9834(00)81487-6 [21] KRITZINGER J A. The role of sulfur in commercial iron-based Fischer-Tropsch catalysis with focus on C2-product selectivity and yield[J]. Catal Today, 2002, 71(3): 307-318. http://www.sciencedirect.com/science/article/pii/S0920586101004576 [22] PENDYALA V R R, JACOBS G, MOHANDAS J C. Fischer-Tropsch synthesis: Effect of water over iron-based catalysts[J]. Catal Lett, 2010, 140(3/4): 98-105. doi: 10.1007/s10562-010-0452-7 [23] ANDERSON R.B. Kinetics of the Fischer-Tropsch synthesis on iron catalysts[J]. Synfacts, 1964, 44(2): 1065-1070. [24] SATTERFIELD C N, HANLON R T, TUNG S E. Effect of water on the iron-catalyzed Fischer-Tropsch synthesis[J]. Ind Eng Chem Prod Res Dev, 1986, 25(3): 407-414. doi: 10.1021/i300023a007 [25] BELL W K, HAAG W O. Conversion of synthesis gas to liquid hydrocarbons gel: US 4978689[P]. 1990-12-18. [26] GALVISl H M T, BITTER J H, DAVIDIAN T. Iron particle size effects for direct production of lower olefins from synthesis gas[J]. J Am Chem Soc, 2012, 134(39): 16207-16215. doi: 10.1021/ja304958u [27] ZHANG H B, SCHRADER G L. Characterization of a fused iron catalyst for Fischer-Tropsch synthesis by in situ laser Raman spectroscopy[J]. J Catal, 1985, 95(1): 325-332. doi: 10.1016/0021-9517(85)90038-7 -





 下载:
下载: