-
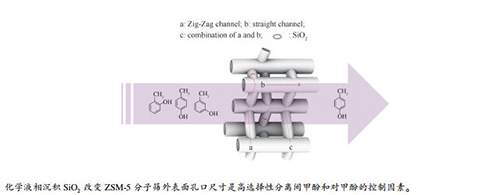
摘要: 利用分子筛择形特点,对煤直接液化油中的混合酚实施高效分离。本研究选取间甲酚和对甲酚作为分离煤直接液化油馏分段混合酚的模型化合物,采用化学液相沉积法对HZSM-5吸附剂的孔口结构进行改变,分析分子筛硅铝比及颗粒粒径对模型化合物间甲酚和对甲酚吸附分离性能的影响,以获得高性能固相吸附剂,并将其应用于180-190℃馏分段混合酚分离。结果表明,当分子筛硅铝比为25、粒径为3-5 μm时,分子筛的孔口结构调节效果最优;当正硅酸乙酯的最小用量为0.2 mL/g时,固相吸附剂的吸附量为0.03 g/g,对甲酚选择性高于95%。由于外表面沉积物对吸附剂的孔口结构变化,导致对甲酚选择性的提高。进一步采用HZSM-5(1)吸附剂对真实煤直接液化油混合酚的分离中发现,苯酚和对甲酚的选择性均达到100%。Abstract: The shape selection features of molecular sieve were used to efficiently separate the mixed phenols of oil fraction from coal direct liquefaction. In this paper, m-cresol and p-cresol were selected as the model compounds for coal liquified oil fraction. The pore structure of HZSM-5 adsorbent was adjusted by chemical liquid phase deposition method. Influence of ratio of silica to alumina and particle size of molecular sieve on the structural properties after modification were investigated. Considering the modified effect on adsorption and separation properties of cresol and p-cresol, a high-performance solid phase adsorbent was obtained, and was applied to separation of phenols in liquid oil from 180-190℃ fraction. The results show that when the molecular sieve has a silica-alumina ratio of 25 and a particle size of 3-5 μm, the pore structure adjustment effect of the molecular sieve is optimal. When the minimum amount of tetraethyl orthosilicate is 0.2 mL/g, the adsorption capacity of solid phase adsorption is 0.03 g/g, and the selectivity of p-cresol is greater than 95%. The selectivity to p-cresol is increased due to changes in the orifice regulation of the adsorbent on the outer surface deposits. Furthermore, using modified HZSM-5(1) adsorbent to separate the mixed phenols from real coal direct liquefied oil, the selectivity of phenol and p-cresol reached 100%.
-
表 1 HZSM-5和HZSM-5(1)吸附剂的比表面积和孔容
Table 1 Specific surface area and pore volume of HZSM-5 and HZSM-5(1) adsorbents
Adsorption BET specific surface area A/(m2·g-1) Total pore volume v/(cm3·g-1) HZSM-5 429.38 0.25 HZSM-5(1) 409.91 0.25 表 2 180-190 ℃馏分段液化油混合酚组成
Table 2 Composition of mixed phenol in 180-190 ℃ distillate of liquefied oil
Sample Mixed phenols /% Experimental value in raffinate /% Calculated value in raffinate /% p-cresol 14.57 0.00 0.00 m-cresol 25.81 37.80 40.79 o-cresol 14.24 23.64 22.50 Phenol 22.14 0.00 0.00 2-ethyl phenol 3.26 7.04 5.15 3-ethyl phenol 3.68 7.26 5.82 4-ethyl phenol 7.25 8.10 11.46 2, 4-dimethylphenol 2.80 5.38 4.42 3, 5-dimethylphenol 6.24 10.37 9.86 Total 100 100 100 -
[1] YU X Y, LIU C H, CHENG W P, YANG J G, HE M Y. Tert-butylation of p-cresol catalyzed by Al-MCM-41 mesoporous molecular sieves[J]. J Fuel Chem Technol, 2006, 34(6):757-760. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb200606024 [2] 包铁竹, 曾凡礼, 曹贤, 李总成. Inventors络合萃取结晶法分离提纯对甲酚工艺: CN, 1127241A[P]. 1996-07-24.BAO Tie-zhu, ZENG Fan-li, CAO Xian, LI Zong-cheng. Study on separation and purification p-cresol by complexing extraction and crystallization: CN, 1127241A[P]. 1996-07-24. [3] 侯颖, 眭贤明, 杨立荣, 吴坚平, 徐刚.尿素一溴化钙联用分离制高纯度间甲酚[J].化学反应工程与工艺, 2011, 27(2):178-182. http://www.cnki.com.cn/Article/CJFDTotal-HXFY201102020.htmHOU Ying, KUI Xian-ming, YANG Li-rong, WU Jian-ping, XU Gang. Preparation of high purity m-cresol by separation of urea and calcium bromide[J]. Chem React Eng Technol, 2011, 27(2):178-182. http://www.cnki.com.cn/Article/CJFDTotal-HXFY201102020.htm [4] 宋晓敏, 陈源光.间甲酚的分离精制[J].现代化工, 1997, 17(6):2829. http://d.old.wanfangdata.com.cn/Periodical/trqhg201601019SONG Xiao-min, CHEN Yuan-guang. Separation and purification of m-cresol[J]. Mod Chem Ind, 1997, 17(6):2829. http://d.old.wanfangdata.com.cn/Periodical/trqhg201601019 [5] 王春蓉.烃化法分离间/对甲酚的研究[J].应用化工, 2009, 38(8):1196-1198. doi: 10.3969/j.issn.1671-3206.2009.08.033WANG Chun-rong. Study on the separation of meta-and para-cresol with alkyl product[J]. Appl Chem Ind, 2009, 38(8):1196-1198. doi: 10.3969/j.issn.1671-3206.2009.08.033 [6] LIU X M, ZHOU J X, GUO X W, LIU M, MA X L, SONG C S, WANG C. SO3H-functionalized Ionic liquids for selective alkylation of p-cresol with tert-butanol[J]. Ind Eng Chem Res, 2008, 47(15):5298-5303. doi: 10.1021/ie070647t [7] ZARETSKIJ M I, YAKOVLEV I P, TYRLOV A A, CHARTOV E M. Extractive separation of isomers of cresol in fluid-fluid system[J]. Koks Khim, 2002, (3):30-33. [8] SHIAU L D, HUANG C H, LIU K F. Separation of the cresol isomers by stripping crystallization[J]. Asia-Pac J Chem Eng, 2012, 7(S1):S26-S31. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1002/apj.629 [9] TOMITA T, SUZUKI K, SATOMI Y, HIRAMINE T. Method for separation p-cresol: JP, 2007137787[P]. 2007-03-14. [10] NAMBA S, KANAI Y, SHOJI H, YASHIMA T. Separation of p-isomers from disubstituted benzenes by means of shape-selective adsorption on mordenite and ZSM-5 zeolites[J]. Zeolites, 1984, 4(1):77-80. doi: 10.1016/0144-2449(84)90078-2 [11] 乐英红, 唐颐, 高滋.化学液相沉积法精细调变沸石孔径[J].石油学报(石油加工), 1997, 13(1):30-35. http://www.cnki.com.cn/Article/CJFDTotal-SXJG701.005.htmLE Ying-hong, TANG Yi, GAO Zi. Fine modulated zeolite pore size by chemical liquid phase deposition[J]. Acta Pet Sin (Pet Process Sect), 1997, 13(1):30-35. http://www.cnki.com.cn/Article/CJFDTotal-SXJG701.005.htm [12] NEUZIL R W, ROSBACK D H. Process for the separation of cresol isomers: US, 3969422[J]. 1976-07-13. [13] VIJAYAKUMAR J, CHIKKALA S K, MANDAL S, MAYADEVI S. Adsorption of cresols on zinc-aluminium hydroxides-A comparison with zeolite-X[J]. Sep Sci Technol, 2011, 46(3):483-488. doi: 10.1080/01496395.2010.510494 [14] YUE Y H, TANG Y, LIU Y, GAO Z. Chemical liquid deposition zeolites with controlled pore-opening size and shape-selective separation of isomers[J]. Ind Eng Chem Res, 1996, 35(2):430-433. http://cn.bing.com/academic/profile?id=ea5340a029b3bc5eedd91f279f7d8de8&encoded=0&v=paper_preview&mkt=zh-cn [15] 乐英红, 唐颐, 阚勇志, 高滋.化学液相沉积法调变沸石孔径及异构体择形分离[J].化学学报, 1996, 54(6):591-597. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxxb199606012LE Ying-hong, TANG Yi, KAN Yong-zhi, GAO Zi. Pore size control of NaY zeolite by chemical liquid deposition and shape-selective separation[J]. Acta Chim Sin, 1996, 54(6):591-597. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxxb199606012 [16] LEE K R, TAN C S. Separation of m-and p-cresols in compressed propane using modified HZSM-5 pellets[J]. Ind Eng Chem Res, 2000, 39(4):1035-1038. doi: 10.1021/ie990613o [17] 高振楠, 杜淑凤, 李文博, 李克健.煤炭直接液化产品油碱洗提酚过程研究[J].煤炭学报, 2009, 34(10):1383-1387. doi: 10.3321/j.issn:0253-9993.2009.10.017GAO Zhen-nan, DU Shu-feng, LI Wen-bo, LI Ke-jian. Study on caustic washing process for extracting phenolics direct coal liquefaction product distillate[J]. J China Coal Soc, 2009, 34(10):1383-1387. doi: 10.3321/j.issn:0253-9993.2009.10.017 [18] EMEIS C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J]. J Catal, 1993, 141(2):347-354. doi: 10.1006-jcat.1993.1145/ [19] MITSUYOSHI D, KUROIWA K, KATAOKA Y, NAKAGAWA T, KOSAKA M, NAKAMURA K, SUGANUMA S, ARAKI Y, KATADA N. Shape selectivity in toluene disproportionation into para-xylene generated by chemical vapor deposition of tetramethoxysilane on MFI zeolite catalyst[J]. Microporous Mesoporous Mater, 2017, 242:118-126. doi: 10.1016/j.micromeso.2017.01.022 [20] 江露. CaO-SiO2-P2O5-FeO熔渣结构与粘度的基础研究[D].重庆: 重庆大学, 2015.JIANG Lu. Fundamental research on the structure and viscosity of molten CaO-SiO2-P2O5-FeO Slag[D]. Chongqing: Chongqing University, 2015. [21] IVANDA M, CLASEN R, HORNFECK M, KIEFER W. Raman spectroscopy on SiO2 glasses sintered from nanosized particles[J]. J Non-Crys Solids, 2003, 322:46-52. doi: 10.1016/S0022-3093(03)00172-8 [22] 郝天亮, 陈钢进. SiO2薄膜的微观结构与驻极体特性的相关性研究[J].功能材料, 2014, 45(22):22091-22095. doi: 10.3969/j.issn.1001-9731.2014.22.019HAO Tian-liang, CHEN Gang-jin. Study on relations between microstructures and electret properties of SiO2 film[J]. J Funct Mater, 2014, 45(22):22091-22095. doi: 10.3969/j.issn.1001-9731.2014.22.019 -





 下载:
下载: