Modeling study on the biomass char gasification kinetics under CO2 atmosphere: Ⅱ. Pre-exponential factor
-
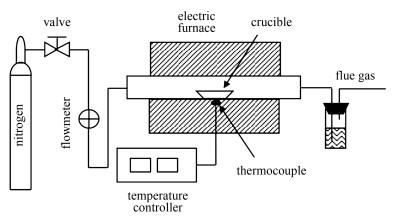
摘要: 基于简单碰撞理论,建立了生物质焦炭CO2气化反应速率的计算方法,找出了表征指数前因子大小的关键组合参数。在此基础上,对六种生物质及其脱灰焦炭的物理化学特性进行了检测分析,利用热重分析仪在800-1000 ℃对各种生物质进行了CO2等温气化实验,将得到的指数前因子实验数据与模型分析结果进行对比。研究表明,指数前因子与构建的组合参数之间存在较好的相关性,建立的通用关系式可为气化反应规律的进一步阐明提供有益的参考。Abstract: Based on the simple collision theory (SCT), the calculation method of biomass char gasification rate was developed and the combined parameters to characterize the pre-exponential factor were found. Furthermore, some experimental tests for six acid-washed biomass chars, such as the isothermal gasification and so on, were performed under CO2 atmosphere, using a thermo-gravimetric analyzer (TGA) over the temperature ranges of 800-1000 ℃, respectively. Through the comparison of experimental data and modeling results, it is found that a good agreement is made and the developed model equations can provide an effective guidance to clarify the general gasification law of biomass chars.
-
Key words:
- biomass char /
- CO2 gasification /
- simple collision theory /
- pre-exponential factor /
- modelling
-
表 1 生物质样品的工业分析和元素分析
Table 1 Proximate analysis and ultimate analysis of samples
Biomass sample Proximate analysis wd/% Ultimate analysis wd/% Average molecular weight /(g·mol-1) V FC A C H N S O Mv SS-de 55.45 10.66 33.89 31.41 4.45 1.01 0.29 28.95 43.5 ML-de 68.66 17.11 14.23 40.57 5.05 1.60 0.24 38.31 45.5 PS-de 80.38 15.54 4.08 46.05 6.16 0.12 0.06 43.53 60.7 CC-de 73.86 16.43 9.71 41.09 5.69 0.49 0.08 42.94 50.6 RH-de 65.64 16.02 18.34 37.55 5.41 1.05 0.18 37.47 40.8 BS-de 68.27 24.59 7.14 43.19 5.97 0.97 0.07 42.66 31.5 表 2 生物质焦炭的无机元素分析
Table 2 Inorganic component analysis of biomass chars
Char sample Content wd/% K Na Ca Mg SS char 1.638 0.023 1.870 1.067 ML char 1.102 0.005 13.11 0.600 PS char 0.462 0.003 0.737 0.139 CC char 2.112 0.010 0.576 0.430 RH char 0.785 0.078 0.401 0.136 BS char 4.831 0.057 0.513 0.251 SS-de char 0.167 0.012 0.092 0.065 ML-de char 0.075 0.003 0.054 0.019 PS-de char 0.070 0.002 0.170 0.031 CC-de char 0.176 0.015 0.099 0.080 RH-de char 0.053 0.008 0.020 0.014 BS-de char 0.106 0.010 0.056 0.017 表 3 焦样的粒径分析
Table 3 Particle size analysis of biomass chars
Char sample Particle size d/μm median diameter area-averaged diameter volume-averaged diameter SS-de char 26.006 19.019 31.469 ML-de char 22.579 14.604 37.348 PS-de char 49.929 30.655 60.904 CC-de char 25.791 17.601 30.39 RH-de char 8.658 4.72 30.32 BS-de char 20.392 13.951 26.968 表 4 生物质脱灰焦的初始反应速率
Table 4 Initial gasification reaction rate of acid-washed chars
Char sample rc0 (1/min) 800 ℃ 850 ℃ 900 ℃ 925 ℃ 950 ℃ 975 ℃ 1 000 ℃ SS-de char 0.002 8 0.007 5 0.033 0 0.049 6 0.107 3 0.140 2 0.231 0 ML-de char 0.001 0 0.003 3 0.008 2 0.013 2 0.023 5 0.039 0 0.066 8 PS-de char 0.008 1 0.025 4 0.064 0 0.093 0 0.153 1 0.287 4 0.350 0 CC-de char 0.001 2 0.004 0 0.015 9 0.028 2 0.042 0 0.073 0 0.124 2 RH-de char 0.000 9 0.001 8 0.006 9 0.013 0 0.016 8 0.034 6 0.058 7 BS-de char 0.000 8 0.002 3 0.008 7 0.013 1 0.024 6 0.042 9 0.062 9 表 5 孔隙平均长度与焦炭表观直径的比例系数β
Table 5 Ratio of average pore length to char apparent diameter for different acid-washed chars
Char sample β (m/m) 800 ℃ 850 ℃ 900 ℃ 925 ℃ 950 ℃ 975 ℃ 1 000 ℃ SS-de char 2.34×10-3 1.34×10-3 1.43×10-3 1.11×10-3 1.27×10-3 9.02×10-4 8.28×10-4 ML-de char 1.49×10-3 1.01×10-3 6.19×10-4 5.13×10-4 4.84×10-4 4.37×10-4 4.16×10-4 PS-de char 5.28×10-3 3.52×10-3 2.16×10-3 1.62×10-3 1.41×10-3 1.44×10-3 9.77×10-4 CC-de char 1.37×10-3 9.96×10-4 9.53×10-4 8.71×10-4 6.88×10-4 6.50×10-4 6.16×10-4 RH-de char 3.69×10-3 1.55×10-3 1.46×10-3 1.42×10-3 9.71×10-4 1.09×10-3 1.03×10-3 BS-de char 1.40×10-3 8.48×10-4 7.76×10-4 6.03×10-4 6.00×10-4 5.69×10-4 4.64×10-4 -
[1] KIRKELS A F, VERBONG G P J. Biomass gasification: Still promising? A 30-year global overview[J]. Renewable Sustainable Energy Rev, 2011, 15(1): 471-481. doi: 10.1016/j.rser.2010.09.046 [2] KAJITANI S, SUZUKI N, ASHIZAWA M, HARA S. CO2 gasification rate analysis of coal char in entrained flow coal gasifier[J]. Fuel, 2006, 85(2): 163-169. doi: 10.1016/j.fuel.2005.07.024 [3] KIRUBAKARAN V, SIVARAMAKRISHNAN V, NALINI R, SEKAR T, PREMALATHA M, SUBRAMANIAN P. A review on gasification of biomass[J]. Renewable Sustainable Energy Rev, 2009, 13(1): 179-186. doi: 10.1016/j.rser.2007.07.001 [4] FERMOSO J, ARIAS B, PEVIDA C, PLAZA M G, RUBIERA F, PIS J J. Kinetic models comparison for steam gasification of different nature fuel chars[J]. J Thermal Anal Calorimetry, 2008, 91(3): 779-786. doi: 10.1007/s10973-007-8623-5 [5] SEO D K, LEE S K, KANG M W, HWANG J, YU T U. Gasification reactivity of biomass chars with CO2[J]. Biomass Bioenergy, 2010, 34(12): 1946-1953. doi: 10.1016/j.biombioe.2010.08.008 [6] OLLERO P, SERRERA A, ARJONA R, ALCANTARILLA S. The CO2 gasifcation kinetics of olive residue[J]. Biomass Bioenergy, 2003, 24(2): 151-161. doi: 10.1016/S0961-9534(02)00091-0 [7] FOUGA G G, DE MICCO G, BOH A E. Kinetic study of argentinean asphaltite gasification using carbon dioxide as gasifying agent[J]. Fuel, 2011, 90(2): 674-680. doi: 10.1016/j.fuel.2010.09.037 [8] COZZANI V. Reactivity in oxygen and carbon dioxide of char formed in the pyrolysis of refuse-derived fuel[J]. Ind Eng Chem Res, 2000, 39(4): 864-872. doi: 10.1021/ie990534c [9] 米铁, 陈汉平, 唐汝江, 吴创之, 马隆龙, 邵敬爱, 王贤华, 赫俏, 刘德昌, 郑楚光.生物质半焦气化的反应动力学[J].太阳能学报, 2005, 26(6): 766-771. http://cdmd.cnki.com.cn/Article/CDMD-10611-1015969021.htmMI Tie, CHEN Han-ping, TANG Ru-jiang, WU Chuang-zhi, MA Long-long, SHAO Jing-ai, WANG Xian-hua, HE Qiao, LIU De-chang, ZHENG Chu-guang. Reactivity study on biomass chars in CO2 atmosphere[J]. Acta Energ Sol Sin, 2005, 26(6): 766-771. http://cdmd.cnki.com.cn/Article/CDMD-10611-1015969021.htm [10] 黄艳琴, 阴秀丽, 吴创之, 汪丛伟, 谢建军, 周肇秋, 马隆龙, 李海滨.稻秆半焦与CO2气化反应特性的研究[J].燃料化学学报, 2009, 37(3): 289-295. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17445.shtmlHUANG Yan-qin, YIN Xiu-li, WU Chuang-zhi, WANG Cong-wei, XIE Jian-jun, ZHOU Zhao-qiu, MA Long-long, LI Hai-bin. Study on CO2 gasification reactivity of rice straw chars[J]. J Fuel Chem Technol, 2009, 37(3): 289-295. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17445.shtml [11] 赵冰, 周志杰, 丁路, 于广锁.快速热处理石油焦与煤的微观结构变化及气化活性分析[J].燃料化学学报, 2013, 41(1): 40-45. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18098.shtmlZHAO Bing, ZHOU Zhi-jie, DING Lu, YU Guang-suo. Changes in the microstructure and gasification reactivity of petroleum coke and coal samples after rapid pyrolysis[J]. J Fuel Chem Technol, 2013, 41(1): 40-45. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18098.shtml [12] 肖瑞瑞, 陈雪莉, 王辅臣, 于广锁.生物质半焦CO2气化反应动力学研究[J].太阳能学报, 2012, 33(2): 236-242.XIAO Rui-rui, CHEN Xue-li, WANG Fu-chen, YU Guang-suo. Research on kinetics characteristics of gasification biomass semi-char with CO2[J]. Acta Energ Sol Sin, 2012, 33(2): 236-242. [13] 刘文钊, 余剑, 张聚伟, 高士秋, 许光文.多孔物质气固反应动力学研究[J].中国科学:化学, 2012, 42(8): 1210-1216. http://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201208011.htmLIU Wen-zhao, YU Jian, ZHANG Ju-wei, GAO Shi-qiu, XU Guang-wen. Kinetic study of reaction of porous solids[J]. Sci Sin Chim, 2012, 42(8): 1210-1216. http://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201208011.htm [14] FU W B, ZHANG B L, ZHENG S M. A relationship between the kinetic parameters of char combustion and the coal's properties[J]. Combust Flame, 1997, 109(4): 587-598. doi: 10.1016/S0010-2180(97)89632-0 [15] WANG X H, ZENG X J, YANG H L, ZHAO D Q. General modeling and numerical simulation of the burning characteristics of porous chars[J]. Combust Flame, 2012, 159(7): 2457-2465. doi: 10.1016/j.combustflame.2012.02.025 [16] 董存珍, 汪小憨, 曾小军, 邵振华. CO2气氛下生物焦气化反应动力学参数的实验研究: Ⅰ.活化能[J].燃料化学学报, 2014, 42(3): 75-81. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18375.shtmlDONG Cun-zhen, WANG Xiao-han, ZENG Xiao-jun, SHAO Zhen-hua. Experimental study on the gasification kinetic parameters of biomass chars under CO2 atmosphere: Ⅰ. Activation energy[J]. J Fuel Chem Technol, 2014, 42(3): 75-81. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18375.shtml [17] 邵振华, 汪小憨, 曾小军, 吴勇.基于简单碰撞理论的生物质焦炭气化反应的活化能[J].过程工程学报, 2015, 15(4): 599-606. doi: 10.12034/j.issn.1009-606X.215212SHAO Zhen-hua, WANG Xiao-han, ZENG Xiao-jun, WU Yong. Gasification Activation Energy of Biomass Chars Based on Simple Collision Theory[J]. Chin J Process Eng, 2015, 15(4): 599-606. doi: 10.12034/j.issn.1009-606X.215212 [18] 傅培舫, 方庆艳, 姚斌, 周怀春.基于简单碰撞理论煤粉燃烧动力学模型的研究——PART Ⅰ:理论建模与热重实验[J].工程热物理学报, 2005, 26(2): 153-156. http://www.cnki.com.cn/Article/CJFDTOTAL-GCRB200502047.htmFU Pei-fang, FANG Qin-yan, YAO Bin, ZHOU Huai-chun. Study on the reaction kinetics model of pulverized coal combustion based on SCT-part Ⅰ: Theory and TGA[J]. J Eng Thermophy, 2005, 26(2): 153-156. http://www.cnki.com.cn/Article/CJFDTOTAL-GCRB200502047.htm [19] 付鹏, 胡松, 向军, 孙路石, 张安超, 杨涛, 江龙.生物质颗粒孔隙结构在热解过程中的变化[J].化工学报, 2009, 60(7): 1793-1799. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ200907031.htmFU Peng, HU Song, XIANG Jun, SUN Lu-shi, ZHANG An-chao, YANG Tao, JIANG Long. Evolution of pore structure of biomass particles during pyrolysis[J]. CIESC J, 2009, 60(7): 1793-1799. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ200907031.htm [20] YIP K, TIAN F J, HAYASHI J, WU H. Effect of alkali and alkaline earth metallic species on biochar reactivity and syngas compositions during steam gasification[J]. Energy Fuels, 2009, 24(1): 173-181. doi: 10.1021/ef900534n?src=recsys&journalCode=enfuem [21] ZHANG Y, ASHIZAWA M, KAJITANI S, MIURA K. Proposal of a semi-empirical kinetic model to reconcile with gasification reactivity profiles of biomass chars[J].Fuel, 2008, 87(4 /5): 475-481. http://www.sciencedirect.com/science/article/pii/S0016236107002098 [22] 林雄超, 王彩红, 田斌, 张书, 周剑林, 王永刚.脱灰对两种烟煤半焦碳结构及CO2气化反应性的影响[J].中国矿业大学学报, 2013, 42(6): 1040-1046. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGKD201306024.htmLIN Xiong-chao, WANG Cai-hong, TIAN Bin, ZHANG Shu, ZHOU Jian-lin, WANG Yong-gang. Effects of de-ashing on the micro-structural transformation and CO2 reactivity of two Chinese bituminous coal chars[J]. J China Univ Min Technol, 2013, 42(6): 1040-1046. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGKD201306024.htm [23] MIN F F, ZHANG M X, Zhang Y, CAO Y, PAN W P. An experimental investigation into the gasification reactivity and structure of agricultural waste chars[J]. J Analy Appl Pyrolysis, 2011, 92(1): 250-257. doi: 10.1016/j.jaap.2011.06.005 -





 下载:
下载: