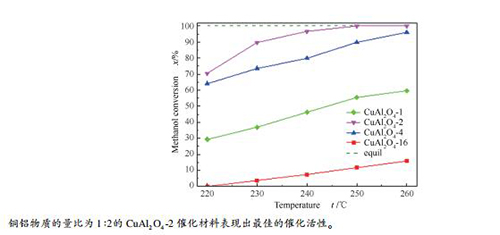
-
摘要: 以γ-Al2O3为原料采用原位合成法制备CuAl2O4催化材料,通过XRF、XRD、BET和H2-TPR等手段对催化材料进行表征,考察铜铝物质的量比对CuAl2O4催化材料结构、性质及其催化甲醇水蒸气重整制氢性能的影响。结果表明,不同铜铝物质的量比主要影响了铜物种的还原性能,从而影响了其催化甲醇水蒸气重整制氢的性能。当铜铝物质的量比为1:2时,CuAl2O4催化材料的催化性能较好,在反应温度为260℃,水醇物质的量比为1.2,甲醇气体空速为800 h-1时,甲醇转化率为100%,产氢速率为895 mL/(kg·s)。Abstract: The CuAl2O4 catalytic material was in-situ synthesized using γ-Al2O3 as raw material. The catalytic material was characterized by XRF, XRD, BET and H2-TPR. The effect of the copper-aluminum molar ratios on the structure and properties of CuAl2O4 spinel catalytic material and its performance in hydrogen production from methanol steam reforming were investigated. The results show that the copper-aluminum molar ratios affects reduction performance of copper species, which affects its performance in catalyzing methanol steam reforming to produce hydrogen. When the copper-aluminum molar ratios is 1:2, CuAl2O4 catalytic material has better catalytic performance. When the reaction temperature is 260℃, with a water-methanol molar ratio of 1.2 and methanol gas hourly space velocity of 800 h-1, the methanol conversion reaches 100%, the hydrogen production rate is 895 mL/(kg·s).
-
Key words:
- CuAl2O4 /
- methanol steam reforming /
- hydrogen /
- in-situ synthesis
-
表 1 CuAl2O4-t催化材料的元素含量
Table 1 Elemental content of CuAl2O4-t catalytic materials
Catalyst Content of element w/% Al /Cu
(mol ratio)Cua Cub Ala Alb Oa Ob CuAl2O4-1 45.9 48.9 21.6 20.6 32.5 30.5 0.99 CuAl2O4-2 34.7 35.1 28.3 29.7 37.0 35.2 1.99 CuAl2O4-4 21.7 22.5 38.6 38.0 39.7 39.5 3.97 CuAl2O4-16 6.7 7.1 48.5 48.2 44.8 44.7 15.97 a: theoretical content;b: actual content 表 2 CuAl2O4-t催化材料和γ-Al2O3的比表面积及孔结构参数
Table 2 Specific surface area and pore structure parameters of CuAl2O4-t catalytic materials and γ -Al2O3
Catalyst ABET /(m2·g-1) Bore diameter d/nm CuAl2O4-1 18.6 3.42 CuAl2O4-2 26.2 3.04 CuAl2O4-4 57.6 18.14 CuAl2O4-16 89.9 18.15 γ-Al2O3 145.6 17.89 表 3 CuAl2O4-t催化材料还原峰面积及峰面积占比
Table 3 Reduction peak area and area content of CuAl2O4-t catalytic materials
Catalyst Peak area A/(a.u.) Area /% peak α peak β peak γ peak α peak β peak γ CuAl2O4-1 43242 18474 4562 65 28 7 CuAl2O4-2 6430 25594 1737 19 76 5 CuAl2O4-4 6170 9583 3125 33 51 16 CuAl2O4-16 3934 1209 325 72 22 6 表 4 催化材料的产氢速率对比
Table 4 Hydrogen production rate compare of catalytic materials
Catalyst Reaction temperature t/℃ Water/methanol wmol/% GHSV /h-1 H2 production rateb w/(mL·kg-1·s-1) CuAl2O4-1 260 1.2:1 800 633 CuAl2O4-2 260 1.2:1 800 895 CuAl2O4-4 260 1.2:1 800 820 CuO/CeO2-R[18] 240 1.2:1 800 378 CuO/CeO2[20] 280 1.2:1 800 380 CuZnCeZr[21] 240 1.2:1 1200 510 Zn0.5Ce1Zr9Ox[22] 450 1.4:1 1500 808 GHSV: methanol gas hourly space velocity -
[1] LIN L, ZHOU W, GAO R, YAO S, ZHANG X, XU W. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544(7648):80-83. doi: 10.1038/nature21672 [2] CLAUDE L. From hydrogen production by water electrolysis to its utilization in a PEM fuel cell or in a SO fuel cell:Some considerations on the energy efficiencies[J]. Int J Hydrogen Energy, 2016, 41(34):15415-15425. doi: 10.1016/j.ijhydene.2016.04.173 [3] HOSSAIN M A, JEWARATNAM J, GANESAN P. Prospect of hydrogen production from oil palm biomass by thermochemical process-A review[J]. Int J Hydrogen Energy, 2016, 41(38):16637-16655. doi: 10.1016/j.ijhydene.2016.07.104 [4] SA S, SILVA H, BRANDAO L, SOUSA J M, MENDES A. Catalysts for methanol steam reforming-A review[J]. Appl Catal B:Environ, 2010, 99(1/2):43-57. http://cn.bing.com/academic/profile?id=0f5dff1a475a8a50a266f4a590f5c457&encoded=0&v=paper_preview&mkt=zh-cn [5] LYTKINA A A, ZHILYAEVA N A, ERMILOVA M M, OREKHOVA N V, YAROSLAVTSEV A B. Influence of the support structure and composition of Ni-Cu-based catalysts on hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy, 2015, 40(31):9677-9684. doi: 10.1016/j.ijhydene.2015.05.094 [6] HE J P, YANG Z X, ZHANG L, LI Y, PAN L W. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming[J]. Int J Hydrogen Energy, 2017, 42(15):9930-9937. doi: 10.1016/j.ijhydene.2017.01.229 [7] CHOI H J, KANG M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded[J]. Int J Hydrogen Energy, 2007, 32(16):3841-3848. doi: 10.1016/j.ijhydene.2007.05.011 [8] SANCHES S G, FLORES J H, DA SILVA M I P. Cu/ZnO and Cu/ZnO/ZrO2 catalysts used for methanol steam reforming[J]. Mol Catal, 2018, 454:55-62. doi: 10.1016/j.mcat.2018.05.012 [9] DAS D, LLORCA J, DOMINGUEZ M, COLUSSI S, TROVARELLI A, GAYEN A. Methanol steam reforming behavior of copper impregnated over CeO2-ZrO2 derived from a surfactant assisted coprecipitation route[J]. Int J Hydrogen Energy, 2015, 40(33):10463-10479. doi: 10.1016/j.ijhydene.2015.06.130 [10] XU T K, ZOU J, TAO W T, ZHANG S Y, CUI L, ZENG F L, WANG D Z, CAI W J. I Co-nanocasting synthesis of Cu based composite oxide and its promoted catalytic activity for methanol steam reforming[J]. Fuel, 2018, 183:238-244. http://cn.bing.com/academic/profile?id=e99eb6438e874b0cb0cc166cb92b4a62&encoded=0&v=paper_preview&mkt=zh-cn [11] HUANG Y H, WANG S F, TSAI A P, KAMEOKA S. Catalysts prepared from copper-nickel ferrites for the steam reforming of methanol[J]. J Power Sources, 2015, 281:138-145. doi: 10.1016/j.jpowsour.2015.01.168 [12] 覃发玠, 刘雅杰, 庆绍军, 侯晓宁, 高志贤.甲醇制氢铜铝尖晶石缓释催化剂的研究-不同铜源合成的影响[J].燃料化学学报, 2017, 45(12):84-91. http://www.cnki.com.cn/Article/CJFDTotal-RLHX201712011.htmQIN Fa-jie, LIU Ya-jie, QING Shao-jun, HOU Xiao-ning, GAO Zhi-xian. Study on Cu-Al spinel slow-release catalyst for methanol-to-hydrogen production the effect of different copper sources[J]. J Fuel Chem Technol, 2017, 45(12):84-91. http://www.cnki.com.cn/Article/CJFDTotal-RLHX201712011.htm [13] 郗宏娟, 李光俊, 庆绍军, 侯晓宁, 赵金珍, 刘雅杰, 高志贤.固相法合成铜铝尖晶石催化甲醇重整反应[J].燃料化学学报, 2013, 41(8):998-1002. http://www.ccspublishing.org.cn/article/id/100032964XI Hong-juan, LI Guang-jun, QING Shao-jun, HOU Xiao-ning, ZHAO Jin-zhen, LIU Ya-jie, GAO Zhi-xian. Solid-state synthesis of copper-aluminum spinel catalyzed methanol reforming reaction[J]. J Fuel Chem Technol, 2013, 41(8):998-1002. http://www.ccspublishing.org.cn/article/id/100032964 [14] 李光俊, 郗宏娟, 张素红, 谷传涛, 庆绍军, 侯晓宁, 高志贤.尖晶石CuM2O4(M=Al、Fe、Cr)催化甲醇重整反应的特性[J].燃料化学学报, 2012, 40(12):60-65. http://www.ccspublishing.org.cn/article/id/100032833LI Guang-jun, XI Hong-juan, ZHANG Su-hong, GU Chuan-tao, QING Shao-jun, HOU Xiao-ning, GAO Zhi-xian. Characteristics of spinel CuM2O4 (M=Al, Fe, Cr) catalyzed methanol reforming reaction[J]. J Fuel Chem Technol, 2012, 40(12):60-65. http://www.ccspublishing.org.cn/article/id/100032833 [15] YAHIRO H, NAKAYA K, YAMAMOTO T, SAIKI K, YAMAURA H. Effect of calcination temperature on the catalytic activity of copper supported on γ-alumina for the water-gas-shift reaction[J]. Catal Commun, 2006, 7(4):228-231. doi: 10.1016/j.catcom.2005.11.004 [16] XI H J, HOU X N, LIU Y J, QING S J, GAO Z X. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angew Chem Int Edit, 2014, 126:12080-12083. doi: 10.1002/ange.201405213 [17] 苏石龙, 张磊, 张艳, 雷俊腾, 桂建舟, 刘丹, 刘道胜, 潘立卫.千瓦级PEMFC甲醇水蒸气重整制氢过程热力学模拟[J].石油化工高等学校学报, 2015, 28(2):21-25. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhggdxx201502004SU Shi-long, ZHANG Lie, ZHANG Yan, LEI Jun-teng, GUI Jian-zhou, LIU Dan, LIU Dao-sheng, PAN Li-wei. Thermodynamic simulation of hydrogen production process from kilowatt PEMFC methanol steam reforming[J]. J Petro Univ, 2015, 28(2):21-25. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhggdxx201502004 [18] YANG S Q, ZHOU F, LIU Y J, ZHANG L, YU C, WANG H H, TIAN Y, ZHANG C S, LIU D S. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy, 2019, 44(14):7252-7261. doi: 10.1016/j.ijhydene.2019.01.254 [19] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭.稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(2):179-188. http://www.ccspublishing.org.cn/article/id/00984ce6-5d53-4ea0-929f-0c4b80514203YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lie, YANG Zhan-xu. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(2):179-188. http://www.ccspublishing.org.cn/article/id/00984ce6-5d53-4ea0-929f-0c4b80514203 [20] 刘玉娟, 王东哲, 张磊, 王宏浩, 陈琳, 刘道胜, 韩蛟, 张财顺.载体焙烧气氛对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J].燃料化学学报, 2018, 46(8):992-999. http://www.ccspublishing.org.cn/article/id/a6e5d5bf-0e5d-4327-b0f9-f6cde8e4ef32LIU Yu-juan, WANG Dong-zhe, ZHANG Lie, WANG Hong-hao, CHEN Lin, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Effect of carrier roasting atmosphere on CuO/CeO2 catalyst for methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(8):992-999. http://www.ccspublishing.org.cn/article/id/a6e5d5bf-0e5d-4327-b0f9-f6cde8e4ef32 [21] ZHANG L, PAN L W, NI C J, SUN T J, ZHAO S S, WANG S D, WANG A J, HU Y K. CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming[J]. Int J Hydrogen Energy, 2013, 38(11):4397-4406. doi: 10.1016/j.ijhydene.2013.01.053 [22] SONG Q L, MEN Y, WANG J G, LIU S, CHAI S S, AN W, WANG K, LI Y Y, TANG Y H. Methanol steam reforming for hydrogen production over ternary composite ZnyCe1Zr9Ox catalysts[J]. Int J Hydrogen Energy, 2020, 45(16):9592-9602. doi: 10.1016/j.ijhydene.2020.01.175 -





 下载:
下载: