XANES study on effect of acid treatment on sulfur forms in Yima coal and their transformation behavior during pyrolysis
-
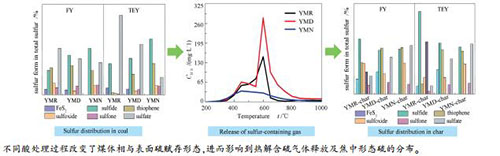
摘要: 利用XANES技术研究了酸处理对义马煤的比表面积、体相及表面硫形态分布和热解过程中硫变迁行为的影响。结果表明,由于酸处理过程中部分镶嵌于有机质中的矿物质被脱除导致部分闭合孔打开,煤的比表面积有所增大。HCl-HF和HCl-HF-HNO3处理脱除了煤中大部分矿物质和无机硫,由于HNO3的强氧化性,YMN中亚砜和砜硫化物的相对含量均高于YMR和YMD。相比煤样体相,酸处理过程对表面形态硫的分布产生了更为明显的影响。酸处理煤样热解含硫气体释放量减少,但由于大部分碱性矿物质的脱除和煤中易分解形态硫相对含量的增加,含硫气体释放率增加。不同形态硫之间的内部转化使得酸处理煤焦中主要形态硫的分布更为均匀。通过HCl-HF-HNO3处理可以有效地脱除煤中矿物质及无机硫,并改变煤中形态硫分布,从而为高灰分、富含黄铁矿的高硫煤的利用提供指导。Abstract: Effect of acid treatment on specific surface area, sulfur forms and their transformation during pyrolysis of Yima coal were investigated by XANES technique. Results show that the specific surface area of acid-treated coals is higher than that of raw coal due to removal of some mosaic minerals in coal matrix and opening of some pores. Most of the minerals and inorganic sulfur are removed by the acid treatment, the relative percentages of sulfide, sulfoxide and sulfone in YMN are higher than that of YMR and YMD due to the higher oxidability of HNO3. Sulfur forms on the coal surface are more modified than that in the coal bulk by the acid treatment. The release amount of sulfur-containing gases during pyrolysis of acid-treated coals decreases, but the accumulated release yields increase due to removal of most alkaline minerals and the relative increase of thermally decomposable sulfur forms in coal. The main sulfur forms show a more even distribution in acid-treated coal chars due to the inter-conversions between different sulfur forms during pyrolysis. HCl-HF-HNO3 process could remove most of the minerals and inorganic sulfur, and change distribution of sulfur forms, which provides a suitable guidance for utilization of high ash, pyrite-rich high-sulfur coals.
-
Key words:
- XANES /
- acid treatment /
- pyrolysis /
- sulfur form /
- transformation behavior
-
表 1 原煤及酸处理煤样分析数据
Table 1 Analysis parameter of YM raw and acid-treated coals
Sample Proximate analysis w/% Ultimate analysis w/% Sulfur form wd /% Mad Ad Vdaf Cdaf Hdaf Ndaf Sd O* Ss Sp So* YMR 6.60 19.64 45.68 75.30 4.80 1.06 1.57 16.88 0.10 0.99 0.48 YMD 6.60 3.70 38.50 78.30 4.34 1.28 1.74 14.27 0.02 0.98 0.74 YMN 7.11 0.59 42.98 78.30 4.16 2.87 0.62 14.05 0.05 0.00 0.57 note: ad is air dried basis; d is dry basis; daf is dry and ash-free basis; Sp is pyritic sulfur; Ss is sulfate sulfur; So is organic sulfur, *: by difference 表 2 原煤灰的成分分析
Table 2 Ash composition of YM raw coal
Sample Ash composition w/% AI SiO2 Al2O3 Fe2O3 CaO MgO TiO2 SO3 K2O Na2O P2O5 YMR 49.49 20.56 12.17 9.29 0.10 1.70 3.28 1.17 0.84 0.66 0.34 表 3 酸处理煤样产率、脱硫率及比表面积
Table 3 ecovery rate, sulfur removal rate and specific surface area before and after acid treatment
Sample w /% η /% Specific surface area
A/(m2·g-1)YMR - - 7.5 YMD 84.78 6.04 24.2 YMN 84.62 66.58 17.3 -
[1] DEMIRBAŞ A. Demineralization and desulfurization of coals via column froth flotation and different methods[J]. Energy Convers Manage, 2002, 43(7):885-895. doi: 10.1016/S0196-8904(01)00088-7 [2] SAHINOGLU E. Cleaning of high pyritic sulfur fine coal via flotation[J]. Adv Powder Technol, 2018, 29(7):1703-1712. doi: 10.1016/j.apt.2018.04.005 [3] 李斌, 杜霞茹, 李庆峰, 张建民, 王洋.灰分对高硫煤热解部分气化硫变迁的影响[J].环境科学, 2004, 25(1):149-153. http://d.old.wanfangdata.com.cn/Periodical/hjkx200401032LI Bin, DU Xia-ru, LI Qing-feng, ZHANG Jian-min, WANG Yang. The effect of minerals on transformation of sulfur during pyrolysis and partial gasification[J]. Environ Sci, 2004, 25(1):149-153. http://d.old.wanfangdata.com.cn/Periodical/hjkx200401032 [4] WANG B, ZHAO S, HUANG Y, ZHANG J. Effect of some natural minerals on transformation behavior of sulfur during pyrolysis of coal and biomass[J]. J Anal Appl Pyrolysis, 2014, 105:284-294. doi: 10.1016/j.jaap.2013.11.015 [5] ZHANG Y, LIANG P, JIAO T, WU J, ZHANG H. Effect of foreign minerals on sulfur transformation in the step conversion of coal pyrolysis and combustion[J]. J Anal Appl Pyrolysis, 2017, 127:240-245. doi: 10.1016/j.jaap.2017.07.028 [6] JIA X, WANG Q, CEN K, CHENG L. Sulfur transformation during the pyrolysis of coal mixed with coal ash in a fixed bed reactor[J]. Fuel, 2016, 177:260-267. doi: 10.1016/j.fuel.2016.03.013 [7] MESROGHLI S, YPERMAN J, JORJANI E, VANDEWIJNGAARDEN J, REGGERS G, CARLEER R, NOAPARAST M. Changes and removal of different sulfur forms after chemical desulfurization by peroxyacetic acid on microwave treated coals[J]. Fuel, 2015, 154:59-70. doi: 10.1016/j.fuel.2015.03.058 [8] TANG L, WANG S, GUO J, TAO X, HE H, FENG L, CHEN S, XU N. Exploration on the removal mechanism of sulfur ether model compounds for coal by microwave irradiation with peroxyacetic acid[J]. Fuel Process Technol, 2017, 159:442-447. doi: 10.1016/j.fuproc.2016.12.019 [9] ZHANG L, LI Z, YANG Y, ZHOU Y, KONG B, LI J, SI L. Effect of acid treatment on the characteristics and structures of high-sulfur bituminous coal[J]. Fuel, 2016, 184:418-429. doi: 10.1016/j.fuel.2016.07.002 [10] 王美君, 付春慧, 常丽萍, 谢克昌.逐级酸处理对锡盟褐煤的结构及热解特性的影响[J].燃料化学学报, 2012, 40(8):906-911. doi: 10.3969/j.issn.0253-2409.2012.08.002WANG Mei-jun, FU Chun-hui, CHANG Li-ping, XIE Ke-chang. Effect of fractional step acid treatment process on the structure and pyrolysis characteristics of Ximeng brown coal[J]. J Fuel Chem Technol, 2012, 40(8):906-911. doi: 10.3969/j.issn.0253-2409.2012.08.002 [11] 张晋玲, 王美君, 陈望舒, 付春慧, 任秀荣, 常丽萍.逐级酸处理对锡盟褐煤的结构及热解特性的影响:气相产物的生成[J].燃料化学学报, 2013, 41(10):1160-1165. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201208002ZHANG Jin-ling, WANG Mei-jun, CHEN Wang-shu, FU Chun-hui, REN Xiu-rong, CHANG Li-ping. Effect of step acid treatment process on the structure and pyrolysis characteristics of Ximeng brown coal:Formation of gaseous products[J]. J Fuel Chem Technol, 2013, 41(10):1160-1165. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201208002 [12] HUFFMAN G P, MITRA S, HUGGINS F E, SHAH N, VAIDYA S, LU F. Quantitative analysis of all major forms of sulfur in coal by X-ray absorption fine structure spectroscopy[J]. Energy Fuels, 1991, 5(4):574-581. doi: 10.1021/ef00028a008 [13] WANG M, LIU L, WANG J, CHANG L, WANG H, HU Y. Sulfur K-edge XANES study of sulfur transformation during pyrolysis of four coals with different ranks[J]. Fuel Process Technol, 2015, 131:262-269. doi: 10.1016/j.fuproc.2014.10.038 [14] WANG M, HU Y, WANG J, CHANG L, WANG H. Transformation of sulfur during pyrolysis of inertinite-rich coals and correlation with their characteristics[J]. J Anal Appl Pyrolysis, 2013, 104(10):585-592. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7cfea0e528f783636a751ecbfb431df5 [15] WANG M, JIA T, WANG J, HU Y, LIU F, WANG H, CHANG L. Changes of sulfur forms in coal after tetrachloroethylene extraction and theirs transformations during pyrolysis[J]. Fuel, 2016, 186:726-733. doi: 10.1016/j.fuel.2016.09.007 [16] 尹艳山, 张轶, 陈厚涛, 刘亮, 鄢晓忠, 陈冬林.高灰煤中矿物质及碳结构的振动光谱分析[J].燃料化学学报, 2015, 43(10):1167-1175. doi: 10.3969/j.issn.0253-2409.2015.10.003YIN Yan-shan, ZHANG Yi, CHEN Hou-tao, LIU Liang, YAN Xiao-zhong, CHEN Dong-lin. Characterization of mineral matters and carbonaceous structure of high-ash coals by vibrational spectroscopy[J]. J Fuel Chem Technol, 2015, 43(10):1167-1175. doi: 10.3969/j.issn.0253-2409.2015.10.003 [17] 孟丽莉, 付春慧, 王美君, 常丽萍.碱金属碳酸盐对褐煤程序升温热解过程中H2S和NH3生成的影响[J].燃料化学学报, 2012, 40(2):138-142. doi: 10.3969/j.issn.0253-2409.2012.02.002MENG LI-li, FU Chun-hui, WANG Mei-jun, CHANG Li-ping. Effect of alkali carbonates on the formation of H2S and NH3 during temperature programmed pyrolysis of brown coal[J]. J Fuel Chem Technol, 2012, 40(2):138-142. doi: 10.3969/j.issn.0253-2409.2012.02.002 [18] 刘诗薇, 邹冲, 赵俊学, 李小明, 折媛, 吴浩, 马成.不同焦煤中硫的赋存形态及热解气体逸出分析[J].煤炭转化, 2018, 41(2):24-30. doi: 10.3969/j.issn.1004-4248.2018.02.004LIU Shi-wei, ZOU Chong, ZHAO Jun-xue, LI Xiao-ming, ZHE Yuan, WU Hao, MA Cheng. Speciation of sulfur and regularity of pyrolysis gas evolution in different coking coals[J]. Coal Convers, 2018, 41(2):24-30. doi: 10.3969/j.issn.1004-4248.2018.02.004 [19] 刘粉荣, 李文, 李保庆, 白宗庆.常压程序升温还原-质谱法研究遵义煤在热解过程中硫的变迁行为[J].燃料化学学报, 2008, 36(1):6-9. doi: 10.3969/j.issn.0253-2409.2008.01.002LIU Fen-rong, LI Wen, LI Bao-qing, BAI Zong-qing. Sulfur transformation during pyrolysis of Zunyi coal by atmosphere pressure-temperature programmed reduction-mass spectrum[J]. J Fuel Chem Technol, 2008, 36(1):6-9. doi: 10.3969/j.issn.0253-2409.2008.01.002 [20] ZHAO H, BAI Z, YAN J, BAI J, LI W. Transformations of pyrite in different associations during pyrolysis of coal[J]. Fuel Process Technol, 2015, 131:304-310. doi: 10.1016/j.fuproc.2014.11.035 [21] 李梅, 杨俊和, 张启锋, 常海洲, 孙慧.用XPS研究新西兰高硫煤热解过程中氮、硫官能团的转变规律[J].燃料化学学报, 2013, 41(11):1287-1293. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201311002LI Mei, YANG Jun-he, ZHANG Qi-feng, CHANG Hai-zhou, SUN Hui. XPS study on transformation of N-and S-functional groups during pyrolysis of high sulfur New Zealand coal[J]. J Fuel Chem Technol, 2013, 41(11):1287-1293. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201311002 [22] LIU L, FEI J, CUI M, HU Y, WANG J. XANES spectroscopic study of sulfur transformations during co-pyrolysis of a calcium-rich lignite and a high-sulfur bituminous coal[J]. Fuel Process Technol, 2014, 121:56-62. doi: 10.1016/j.fuproc.2013.12.008 -





 下载:
下载: