Removal of NO at low temperature and in the absence of ammonia over spherical activated carbon loaded with MnOx-CeO2 and urea
-
摘要: 以MnOx-CeO2为活性组分、以尿素为还原剂,制备了球形活性炭(SAC)担载MnOx-CeO2和尿素的复合催化剂,利用扫描式电子显微镜-能谱仪(SEM-EDS)、X射线衍射(XRD)和低温N2吸附-脱附等对催化剂的物理化学结构进行了表征,考察了该催化剂在低温(30-90 ℃)和没有氨气情况下对NO的选择性催化还原(SCR)性能。结果表明,在30-50 ℃的反应温度下,炭载体的微孔孔容仍是NO吸附与氧化的主要活性位点;而在70-90 ℃的反应温度下,锰铈金属氧化物成为NO吸附与氧化的主要活性位点,从而提高了催化剂的SCR反应活性。当反应温度为90 ℃、空速为6000 h-1、NO和O2浓度分别为0.05%和20%时,担载8% Mn(锰铈物质的量比为1:1)和10%尿素的催化剂的MOx稳态脱除率可达85.6%,实现了在低温、无氨状况下的高效脱硝。Abstract: A complex catalyst was prepared by loading MnOx-CeO2 and urea on spherical activated carbon (SAC) for the selective catalytic reduction (SCR) of NO at low temperature (30-90 ℃) in the absence of ammonia. The complex catalyst was characterized by scanning electron microscopy-energy dispersive spectrum (SEM-EDS), X-ray diffraction (XRD), and N2 sorption. The results show that at 30-50 ℃, the micropores of SAC acts as the main active sites for NO adsorption and reaction, whereas at 70-90 ℃, MnOx-CeO2 turns to be the main active sites for NO adsorption and reaction; the urea-SCR activity is enhanced by MnOx-CeO2 in a wide temperature range. Over the complex catalyst loaded with 8% Mn (the mole ratio of Mn to Ce is 1) and 10% urea, the steady conversion of MOx reaches 85.6% in the absence of ammonia, under the conditions of 90 ℃, 0.05% NO, 20% O2 and a space velocity of 6000 h-1.
-
Key words:
- spherical activated carbon /
- MnOx-CeO2 /
- urea /
- NO /
- selective catalytic reduction
-
表 1 复合催化剂的孔结构参数
Table 1 Pore structural parameters of the catalysts with different urea loadings
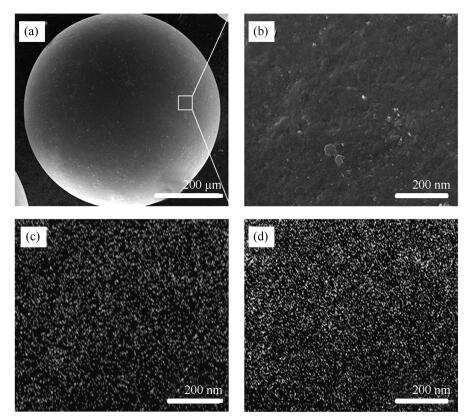
Sample ABETa/(m2·g-1) Amicb/(m2·g-1) vtc/(cm3·g-1) vmicd/(cm3·g-1) d pe /nm SAC 1411 1295 0.62 0.52 0.73 400-8(Mn-Ce)/SAC 710 648 0.32 0.26 0.66 400-8(Mn-Ce)/SAC-5 673 614 0.34 0.25 0.85 400-8(Mn-Ce)/SAC-10 578 528 0.26 0.22 0.67 400-8(Mn-Ce)/SAC-15 476 428 0.22 0.17 0.93 400-8(Mn-Ce)/SAC-20 380 322 0.19 0.13 0.79 a: BET surface area, b: micropore surface area (< 2 nm), c: total pore volume (p/p0=0.996), d: micropore volume (< 2 nm), e: average pore diameter -
[1] BOSCHH, JANSSEN F. Catalytic reduction of nitrogen oxides:A review on the fundamentals and technology[J]. Catal Today, 1988, 2(4):369-532. doi: 10.1016/0920-5861(88)80002-6 [2] ZHANG X, LI X G, WU J S, YANG R C, ZHANG Z H. Selective catalytic reduction of NO by ammonia on V2O5/TiO2 catalyst prepared by sol-gel method[J]. Catal Lett, 2009, 130(1):235-238. http://www.osti.gov/scitech/biblio/22257172 [3] 袁从慧, 刘华彦, 卢晗锋, 李玉芳, 陈银飞.催化氧化-还原吸收法脱除工业含湿废气中MOx[J].环境工程学报, 2008, 2(9):1207-1212. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjwrzljsysb200809012YUAN Cong-hui, LIU Hua-yan, LU Han-feng, LI Yu-fang, CHEN Yin-fei. Catalytic oxidation-reductive absorption process for MOx removal in humid waste gas[J]. Chin J Environ Eng, 2008, 2(9):1207-1212. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjwrzljsysb200809012 [4] SHIRAHAMA N, MOCHIDA I, KORAI Y, CHOI K H, ENJOJI T, SHIMOHARA T, YASUTAKE A. Reaction of NO2 in air at room temperature with urea supported on pitch based activated carbon fber[J]. Appl Catal B:Environ, 2004, 52(3):173-179. doi: 10.1016/j.apcatb.2004.04.003 [5] SHIRAHAMA N, MOCHIDA I, KORAI Y, CHOI K H, ENJOJI T, SHIMOHARA T, YASUTAKE A. Reaction of NO with urea supported on activated carbons[J]. Appl Catal B:Environ, 2005, 57(4):237-245. doi: 10.1016/j.apcatb.2004.04.004 [6] WANG Z, WANG Y L, LONG D H, MOCHIDA I, QIAO W M, ZHAN L, LIU X J, YOON S H, LING L C. Kinetics and mechanism study of low-temperature selective catalytic reduction of NO with urea supported on pitch-based spherical activated carbon[J]. Ind Eng Chem Res, 2011, 50(10):6017-6027. doi: 10.1021/ie102506q [7] WANG Z, WANG Y L, WANG D J, CHEN Q J, QIAO W M, ZHAN L, LING L C. Low-temperature selective catalytic reduction of NO with urea supported on pitch-based spherical activated carbon[J]. Ind Eng Chem Res, 2010, 49(14):6317-6322. doi: 10.1021/ie901772y [8] ZHANG W J, RABIEI S, BAGREEV A, ZHUANG M S, RASOULI F. Study of NO adsorption on activated carbons[J]. Appl Catal B:Environ, 2008, 83(1/2):63-67. https://www.deepdyve.com/lp/elsevier/study-of-no-adsorption-on-activated-carbons-ncyANu6KDH [9] MIYAWAKI J, SHIMOHARA T, SHIMOHARA N, YASUTAKE A, YOSHIKAWA M, MOCHIDA I, YOON S H. Removal of MOx from air through cooperation of the TiO2 photocatalyst and urea on activated carbon fiber at room temperature[J]. Appl Catal B:Environ, 2011, 110:273-278. doi: 10.1016/j.apcatb.2011.09.012 [10] LU P, ZENG Z, LI C T, ZENG G M, GUO J, JIANG X, ZHAI Y B, FAN X P. Room temperature removal of NO by activated carbon fibres loaded with urea and La2O3[J]. Environ Technol, 2012, 33(11):1029-1036. https://www.researchgate.net/profile/Zheng_Zeng5/publication/227855323_Room_temperature_removal_of_NO_by_activated_carbon_fibres_loaded_with_urea_and_La2O3/links/0046352cddb9525fc7000000/Room-temperature-removal-of-NO-by-activated-carbon-fibres-loaded-with-urea-and-La2O3.pdf [11] ZENG Z, LU P, LI C T, ZENG G M, JIANG X, ZHAI Y B, FAN X P. Selective catalytic reduction (SCR) of NO by urea loaded on activated carbon fiber (ACF) and CeO2/ACF at 30℃:The SCR mechanism[J]. Environ Technol, 2012, 33(11):1331-1337. doi: 10.1080/09593330.2011.626799 [12] JIANG X, LU P, LI C T, ZENG Z, ZENG G M, HU L P, MAI L, LI Z. Experimental study on a room temperature urea-SCR of NO over activated carbon fibre-supported CeO2-CuO[J]. Environ Technol, 2013, 34(5):591-598. doi: 10.1080/09593330.2012.707232 [13] 崔华飞, 李彩亭, 路培, 彭敦亮, 郭静, 陈玲. ACF负载金属氧化物及尿素低温去除NO[J].环境科学, 2010, 31(11):2575-2581. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201011005CUI Hua-fei, LI Cai-ting, LU Pei, PENG Dun-liang, GUO Jing, CHEN Ling. Reaction of NO with metal oxides and urea supported on activated carbons at low temperature[J]. Environ sci, 2010, 31(11):2575-2581. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201011005 [14] LIU X J, LIANG X Y, LIU C J, ZHAN L, QIAO W M, LING L C. Pitch spheres stabilized by HNO3 oxidation and their carbonization behavior[J]. New Carbon Mater, 2010, 25(1):29-34. doi: 10.1016/S1872-5805(09)60013-5 [15] 黄增斌, 李翠清, 王振, 徐胜美, 冯凌波, 王虹, 宋永吉, 张伟.不同分子筛负载锰铈催化剂的低温NH3-SCR脱硝性能[J].燃料化学学报, 2016, 44(11):1388-1393. doi: 10.3969/j.issn.0253-2409.2016.11.016HUANG Zeng-bin, LI Cui-qing, WANG Zhen, XU Sheng-mei, FENG Ling-bo, WANG Hong, SONG Yong-ji, ZHANG Wei. Performance of Mn-Ce catalysts supported on different zeolites in the NH3-SCR of MOx[J]. J Fuel Chem Technol, 2016, 44(11):1388-1393. doi: 10.3969/j.issn.0253-2409.2016.11.016 [16] 闫东杰, 玉亚, 黄学敏, 刘树军, 刘颖慧. SO2对Mn-Ce/TiO2低温SCR催化剂的毒化作用研究[J].燃料化学学报, 2016, 44(2):232-238. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18784.shtmlYAN Dong-jie, YU Ya, HUANG Xue-min, LIU Shu-jun, LIU Ying-hui. Poisoning effect of SO2 on Mn-Ce/TiO2 catalysts for NO reduction by NH3 at low temperature[J]. J Fuel Chem Technol, 2016, 44(2):232-238. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18784.shtml [17] 张华伟, 陈江艳, 赵可, 牛庆欣, 王力. Mn/Ce掺杂改性半焦对模拟煤气中单质汞的脱除性能研究[J].燃料化学学报, 2016, 44(4):394-400. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18805.shtmlZHANG Hua-wei, CHEN Jiang-yan, ZHAO Ke, NIU Qing-xin, WANG Li. Removal of vapor-phase elemental mercury from simulated syngas using semi-coke modified by Mn/Cedoing[J]. J Fuel Chem Technol, 2016, 44(4):394-400. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18805.shtml [18] 杨晓燕, 沈伯雄, 马宏卿, 刘亭, 左琛.前驱物对Mn-Ce/Ti-PILC低温SCR脱硝的影响[J].燃料化学学报, 2012, 40(1):119-123. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17871.shtmlYANG Xiao-yan, SHEN Bo-xiong, MA Hong-qing, LIU Ting, ZUO Chen. Study on the effect of Mn-Ce/Ti-PILC on low temperature SCR activity prepared with different precursors[J]. J Fuel Chem Technol, 2012, 40(1):119-123. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17871.shtml [19] WANG J T, WANG M, LI W C, QIAO W M, LONG D H, LING L C. Application of polyethylenimine-impregnated solid adsorbents for direct capture of low-concentration CO2[J]. AIChE J, 2015, 61(3):972-980. doi: 10.1002/aic.v61.3 -





 下载:
下载: