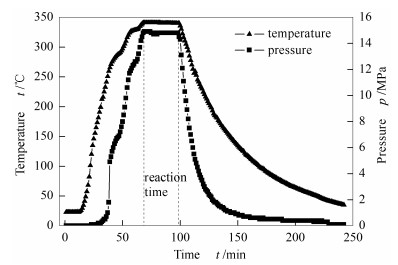
-
摘要: 以玉米秸秆为原料, 添加分子筛催化剂在体积为500 mL的高温高压反应釜中进行催化液化制备生物油实验研究。选取反应温度、催化剂含量和反应时间三个主要因素为变量, 探究其对玉米秸秆催化液化产物分布的影响。利用气相色谱-质谱联用仪(GC-MS) 和傅里叶红外光谱仪(FT-IR) 对玉米秸秆生物油的成分和官能团结构进行分析。结果表明, 玉米秸秆的最佳催化液化条件为, 反应温度为340 ℃, 玉米秸秆15 g, FeHZSM-5催化剂含量为6.67%, 反应时间为30 min。在此条件下, 生物油产率为28.03%, 催化液化整体转化率为81.73%。生物油的主要成分为酚类和长链酯类, 生物油的热值达30.08 MJ/kg。Abstract: The hydrothermal catalytic liquefaction of corn stalk (CS) in a 500 mL batch reactor was conducted. The influence of reaction temperature and catalyst quantity as well as reaction time on the products distribution was investigated. The main components and functional groups of bio-oil with and without catalysts were characterized by gas chromatography/mass spectrometry (GC-MS) and Fourier transform infrared spectrometry (FT-IR). The result shows that the optimal conditions of liquefaction are 15 g CS with 6.67% FeHZSM-5 at 340 ℃ for 30 min, at which the bio-oil yield reaches a maximum of 28.03% with the highest CS conversion of 81.73%. The main components of bio-oil are phenols and esters, and the HHV of the bio-oil reaches to 30.08 MJ/kg.
-
Key words:
- corn stalk /
- catalyst /
- catalytic liquefaction /
- bio-oil
-
表 1 玉米秸秆的工业分析与元素分析
Table 1 Proximate, ultimate and heating value analyses of CS material
Proximate analysis wd/% Ultimate analysis wd/% Heating value Q/(MJ·kg-1) M A V FC C H Oa N S 5.45 5.84 74.55 14.16 41.44 5.31 52.27 0.84 0.14 12.25 a: by difference 表 2 生物油主要组分的GC-MS分析
Table 2 Major chemical compositions of bio-oil
RT. M.F. Compound Area /% Time t/min M.F. Compound Area /% 4.602 C6H8O 2-cyclopente n-1-one, 2-methyl- 0.42 20.436 C19H38 1-nonadecene 1.10 6.428 C6H8O 2-cyclopenten-1-one, 3-methyl- 0.28 20.972 C11H13N 1H-indole, 5, 6, 7-trimethyl- 2.02 9.086 C6H6O phenol 0.77 21.383 C11H13N 2, 3, 7-trimethylindole 1.92 9.870 C7H10O 2-cyclopenten-1-one, 0.58 21.851 C12H15N 1, 2, 3, 7-tetramethylindole 0.89 2, 3-dimethyl- 11.391 C8H12O 2-cyclopenten-1-one, 0.28 22.798 C19H38 1-nonadecene 1.11 2, 3, 4-trimethyl- 12.375 C8H12O 2-cyclopenten-1-one, 2-methyl- 0.40 23.247 C13H26 cyclohexane, 2-butyl- 0.96 1, 1, 3-trimethyl- 12.595 C7H8O2 phenol, 2-methoxy- 0.68 23.362 C15H32O 1-dodecanol, 3, 7, 11-trimethyl- 1.58 12.796 C8H12O 2-cyclopenten-1-one, 0.74 24.902 C19H38 1-nonadecene 2.06 3, 5, 5-trimethyl- 13.475 C7H8O phenol, 4-methyl- 0.86 24.997 C17H34O2 hexadecanoic acid, methyl ester 0.86 13.733 C8H12O 2-cyclopenten-1-one, 0.80 25.102 C16H22O4 dibutyl phthalate 1.81 2, 3, 4-trimethyl- 14.881 C8H10O phenol, 3-ethyl- 0.48 25.227 C16H30O2 9-hexadecenoic acid 2.01 15.072 C8H10O phenol, 2, 5-dimethyl- 0.59 25.351 C16H32O2 n-hexadecanoic acid 2.65 15.321 C8H10O phenol, 4-ethyl- 6.63 25.600 C18H36O2 hexadecanoic acid, ethyl ester 8.71 15.684 C9H10N2 1H-benzimidazole, 5, 6-dimethyl- 0.84 25.762 C19H36O2 10-octadecenoic acid, methyl ester 1.71 15.789 C10H10O benzofuran, 4, 7-dimethyl- 0.42 26.728 C19H36O2 16-octadecenoic acid, methyl ester 1.17 15.866 C10H10O benzofuran, 4, 7-dimethyl- 1.40 26.891 C19H38O2 heptadecanoic acid, 1.09 16-methyl-, methyl ester 16.621 C9H12O phenol, 2, 4, 6-trimethyl- 1.12 26.996 C18H34O2 oleic acid 1.09 16.679 C9H12O phenol, 3-(1-methylethyl)- 1.12 27.244 C18H36O2 octadecanoic acid 10.78 16.879 C9H8O 1H-inden-1-one, 2, 3-dihydro- 0.71 27.598 C20H40O2 octadecanoic acid, ethyl ester 4.51 17.032 C9H12O2 phenol, 4-ethyl-2-methoxy- 2.84 27.694 C17H36O 1-hexadecanol, 2-methyl- 1.39 17.501 C10H10O 1H-inden-1-one, 2, 0.65 27.780 C26H54 octadecane, 1.15 3-dihydro-3-methyl- 3-ethyl-5-(2-ethylbutyl)- 17.606 C11H12O 1H-inden-1-one, 2, 1.02 28.487 C19H38O3 octadecanoic acid, 1.05 3-dihydro-3, 3-dimethyl- 4-hydroxy-, methyl ester 18.199 C8H10O3 phenol, 2, 6-dimethoxy- 1.85 28.545 C22H44 1-docosene 1.05 18.476 C10H14O2 phenol, 2-methoxy-4-propyl- 1.08 28.621 C16H34O 1-decanol, 2-hexyl- 1.17 19.021 C12H14O 1(2H)-naphthalenone, 1.32 29.377 C26H52 9-hexacosene 0.94 5-ethyl-3, 4-dihydro- 19.384 C11H16O2 3-tert-butyl-4-hydroxyanisole 0.80 30.161 C22H44 1-docosene 0.83 19.566 C9H12O3 phenol, 4-methoxy- 2.17 32.513 C30H50 squalene 0.86 3-(methoxymethyl)- 20.149 C10H11N indolizine, 2, 5-dimethyl- 1.56 33.928 C29H48 stigmasta-4, 22-diene 0.37 -
[1] DURAK H, AYSU T. Effects of catalysts and solvents on liquefaction of Onopordum heteracanthum for production of bio-oils[J]. Bioresour Technol, 2014, 166: 309-317. doi: 10.1016/j.biortech.2014.05.051 [2] TOOR S S, ROSENDAHL L, RUDOLF A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies[J]. Energy, 2011, 36(5): 2328-2342. doi: 10.1016/j.energy.2011.03.013 [3] 石阳.农作物秸秆综合利用研究及其在滨州市的示范应用[D].济南:山东大学, 2012.SHI Yang. The comprehensive utilization of crop straw and in Binzhou demonstration application[D]. Jinan: Shandong University, 2012. [4] BILLER P, ROSS A B. Hydrothermal processing of algal biomass for the production of biofuels and chemicals[J]. Biofuels, 2012, 3(5): 603-623. doi: 10.4155/bfs.12.42 [5] HUANG H J, YUAN X Z. Recent progress in the direct liquefaction of typical biomass[J]. Prog Energy Combust Sci, 2015, 49: 59-80. doi: 10.1016/j.pecs.2015.01.003 [6] HUANG H J, YUAN X Z, ZHU H N, LI H, LIU Y, WANG X L, ZENG G M. Comparative studies of thermochemical liquefaction characteristics of microalgae, lignocellulosic biomass and sewage sludge[J]. Energy, 2013, 56(5): 52-60. http://d.scholar.cnki.net/detail/SJESTEMP_U/SJES15122600379301 [7] NO S Y. Application of bio-oils from lignocellulosic biomass to transportation, heat and power generation-A review[J]. Renew Sust Energy Rev, 2014, 40: 1108-1125. doi: 10.1016/j.rser.2014.07.127 [8] 徐玉福, 俞辉强, 朱利华, 王开朝, 崔征, 胡献国.小球藻粉水热催化液化制备生物油[J].农业工程学报, 2012, 28(19): 194-199. http://www.cnki.com.cn/Article/CJFDTOTAL-NYGU201219027.htmXU Yu-fu, YU Hui-qiang, ZHU Li-hua, WANG Kai-chao, CUI Zheng, HU Xian-guo. Preparation of bio-fuel from Chlorella pyrenoidosa by hydrothermal catalytic liquefaction[J]. Trans Chin Soc Agric Eng, 2012, 28(19): 194-199. http://www.cnki.com.cn/Article/CJFDTOTAL-NYGU201219027.htm [9] 程明杨, 袁兴中, 曾光明, 佟婧怡, 王立华, 李辉, 丁艳敏.金属改性催化剂对松木屑液化效果的影响[J].农业工程学报, 2009, 25(8): 210-214. http://www.cnki.com.cn/Article/CJFDTOTAL-NYGU200908041.htmCHENG Ming-yang, YUAN Xing-zhong, ZENG Guang-ming, TONG Jing-yi, WANG Li-hua, LI-Hui, DING Yan-min. Effect of metal-modified catalyst on liquefaction of pine sawdust [J]. Trans Chin Soc Agric Eng, 2009, 25(8): 210-214. http://www.cnki.com.cn/Article/CJFDTOTAL-NYGU200908041.htm [10] LI J H, WANG G M, GAO C L, LV X, WANG Z H, LIU H C.Deoxy-liquefaction of Laminaria japonica to high-quality liquid oil over metal modified ZSM-5 catalysts[J].Energy Fuels, 2013, 27(9): 5207-5214. http://d.scholar.cnki.net/detail/SJCSTEMP_U/SJCS13110600031677 [11] ZHONG C L, WEI X M. A comparative experimental study on the liquefaction of wood[J]. Energy, 2004, 29(11): 1731-1741. doi: 10.1016/j.energy.2004.03.096 [12] 于树峰, 仲崇立.农作物废弃物液化的实验研究[J].燃料化学学报, 2005, 33(2): 206-210. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract16545.shtmlYU Shu-feng, ZHONG Chong-li. Experimental study on liquefaction of agricultural residue[J]. J Fuel Chem Technol, 2005, 33(2): 206-210. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract16545.shtml [13] LI J H, WANG G M, WANG Z H, ZHANG L X, WANG C, YANG Z Y. Conversion of Enteromorpha prolifera to high-quality liquid oil via deoxy-liquefaction[J].J Anal Appl Pyrolysis, 2013, 104: 494-501. doi: 10.1016/j.jaap.2013.05.021 [14] 常胜, 赵增立, 张伟, 郑安庆, 吴文强, 李海滨.不同种类生物油化学组成结构的对比研究[J].燃料化学学报, 2011, 39(10): 746-753. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17815.shtmlCHANG Sheng, ZHAO Zeng-li, ZHANG Wei, ZHENG An-qing, WU Wen-qiang, LI Hai-bin. Comparison of chemical composition and structure of different kinds of bio-oils[J]. J Fuel Chem Technol, 2011, 39(10): 746-753. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17815.shtml [15] MURATA K, HIRANO Y, SAKATA Y, UDDIN M A. Basic study on a continuous flow reactor for thermal degradation of polymers[J]. J Anal Appl Pyrolysis, 2002, 65(1): 71-90. doi: 10.1016/S0165-2370(01)00181-4 -





 下载:
下载: